��Ŀ����
��7�֣� ȼ�ϵ�ȼ�����������ķ�չ�������൱��Ҫ�����á�
��1��������������ģʹ��ȼ�ϵĴ���˳��ľ���ľ̿��ú��ʯ�͡���Ȼ����
������ȼ�������ڻ�ʯȼ�ϵ��� ����Щ��ʯȼ������ ��Դ�����������������������������ȼ����ͨ������Ϊ�������Դ������ ��
����Ȼ������Ҫ�ɷ��ǣ��ѧʽ�� ������ȫȼ�յĻ�ѧ����ʽΪ�� ��
��2��Ϊ�����ú��ȼ�������ʣ�ͨ����ú�Ƴɷ���״��Ŀ���� ��
��3���ִ�����������������Խ��Խ��ѧ��Ӧ�ṩ�������Ѳ���������������������������úͿ�������Դ���� ������һ����
��1��������������ģʹ��ȼ�ϵĴ���˳��ľ���ľ̿��ú��ʯ�͡���Ȼ����
������ȼ�������ڻ�ʯȼ�ϵ��� ����Щ��ʯȼ������ ��Դ�����������������������������ȼ����ͨ������Ϊ�������Դ������ ��
����Ȼ������Ҫ�ɷ��ǣ��ѧʽ�� ������ȫȼ�յĻ�ѧ����ʽΪ�� ��
��2��Ϊ�����ú��ȼ�������ʣ�ͨ����ú�Ƴɷ���״��Ŀ���� ��
��3���ִ�����������������Խ��Խ��ѧ��Ӧ�ṩ�������Ѳ���������������������������úͿ�������Դ���� ������һ����
(1) ��ú����ʯ�͡���Ȼ����������������Ȼ��
��CH4��CH4+2O2 CO2+ 2H2O
CO2+ 2H2O
(2)�����������ĽӴ������ʹ����ȼ��
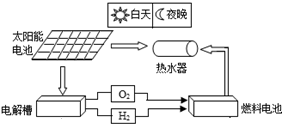
��3��̫���ܵȣ��������֣�
��CH4��CH4+2O2
 CO2+ 2H2O
CO2+ 2H2O(2)�����������ĽӴ������ʹ����ȼ��
��3��̫���ܵȣ��������֣�
����1��ú��ʯ�͡���Ȼ�����ڻ�ʯȼ�ϣ����ڲ���������Դ����Ȼ��ȼ�յ��������Ƕ�����̼��ˮ������Ⱦ��������һ�������Դ���ʴ�Ϊ��ú��ʯ�͡���Ȼ����������������Ȼ����
��2����Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽ��CH4������������ڵ�ȼ�����������ɶ�����̼��ˮ�����CH4��CH4+2O2 CO2+ 2H2O��
CO2+ 2H2O��
��3����ú�Ƴɷ���״��Ŀ��������ú�������ĽӴ�������ٽ�ȼ�գ��ʴ�Ϊ������ú�������ĽӴ������ʹ֮���ȼ�գ�
��4�������������úͿ���������Դ��̫���ܡ������ܡ����ܡ���ϫ�ܵȣ��ʴ�Ϊ��̫���ܵȣ�
��2����Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽ��CH4������������ڵ�ȼ�����������ɶ�����̼��ˮ�����CH4��CH4+2O2
 CO2+ 2H2O��
CO2+ 2H2O����3����ú�Ƴɷ���״��Ŀ��������ú�������ĽӴ�������ٽ�ȼ�գ��ʴ�Ϊ������ú�������ĽӴ������ʹ֮���ȼ�գ�
��4�������������úͿ���������Դ��̫���ܡ������ܡ����ܡ���ϫ�ܵȣ��ʴ�Ϊ��̫���ܵȣ�

��ϰ��ϵ�д�
�����Ŀ