��Ŀ����
��2011?������һģ��һЩ��������Ϊ��ıȡ������Ӥ���̷��������˴��������谷�����Ӻ������������谷��C3H6N6����һ�֢ٴ���ɫ���壬��ζ���ܶ�Ϊ1.573g/cm3��������ˮ����ѹ���۵�Ϊ354�棻�۸���ʱ�ֽ⣻���������ᡢ��������ʷ�����Ӧ���������谷�Σ�
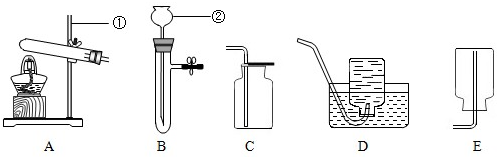
��1�����������У����������谷�������ʵ���
��2����Ҫʹ�����谷������ѧ�仯���ɲ��õķ�����
��3�������谷��Ħ��������
��1�����������У����������谷�������ʵ���
�٣���
�٣���
������ţ�����2����Ҫʹ�����谷������ѧ�仯���ɲ��õķ�����
���¼��Ȼ������ᡢ����ȷ�Ӧ���������谷��
���¼��Ȼ������ᡢ����ȷ�Ӧ���������谷��
����3�������谷��Ħ��������
126g/mol
126g/mol
��1mol�����谷��C3H6N6���к�15
15
molԭ�ӣ���������1�������������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ����н��
��2���������е���Ϣ���з�����������������ʵ��ص���з�������⣻
��3������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ������������Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ��������н��
��2���������е���Ϣ���з�����������������ʵ��ص���з�������⣻
��3������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ������������Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ��������н��
����⣺��1�������������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ��ɴ˿�֪���������谷�������ʵ��Ǣ٢ڣ�
��2���������е���Ϣ���۸���ʱ�ֽ⣻���������ᡢ��������ʷ�����Ӧ���������谷�Σ��ɴ˿�֪Ҫʹ�����谷������ѧ�仯���ɲ��õķ����ǣ����¼��Ȼ������ᡢ����ȷ�Ӧ���������谷�Σ�
��3������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ����������֪�����谷��Ħ������Ϊ��12��3+6+14��6=126g/mol�� ���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɵ�C3H6N6��̼���⡢��ԭ�ӵĸ����ܺ�Ϊ3+6+6=15������1mol�����谷��C3H6N6���к���15molԭ�ӣ�
�ʴ�Ϊ��
��1���٢ڣ�
��2�����¼��Ȼ������ᡢ����ȷ�Ӧ���������谷�Σ�
��3��126g/mol��15��
��2���������е���Ϣ���۸���ʱ�ֽ⣻���������ᡢ��������ʷ�����Ӧ���������谷�Σ��ɴ˿�֪Ҫʹ�����谷������ѧ�仯���ɲ��õķ����ǣ����¼��Ȼ������ᡢ����ȷ�Ӧ���������谷�Σ�
��3������1Ħ�κ����ʵ����������Կ�Ϊ��λ����ֵ�ϵ��ڸ���ԭ�ӵ����ԭ����������֪�����谷��Ħ������Ϊ��12��3+6+14��6=126g/mol�� ���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɵ�C3H6N6��̼���⡢��ԭ�ӵĸ����ܺ�Ϊ3+6+6=15������1mol�����谷��C3H6N6���к���15molԭ�ӣ�
�ʴ�Ϊ��
��1���٢ڣ�
��2�����¼��Ȼ������ᡢ����ȷ�Ӧ���������谷�Σ�
��3��126g/mol��15��
���������⿼��ѧ�������ʵ��������ʣ���ѧ���ʵ����⣬���ܸ������ʵĻ�ѧʽ���м�����Ħ�������Ľ���������

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ