��Ŀ����
Ԫ����(Na2SO4)����������ӡȾ֯��Ĵ�Ⱦ���������ʵ���������һ����3%-5%��Χ��
(1)Ԫ�������ơ���Ԫ�ص�������Ϊ_____����д����������ȣ�
(2)����1000g���ʵ���������Ϊ4%�Ĵ�Ⱦ������Ҫ_____mL(������С�����һλ��������������Ϊ20%����������Һ����Ҫˮ������Ϊ_____g������֪������������������Ϊ20%����������Һ�ܶ�Ϊ1.2g/cm3��
(3)��ij��Ⱦ����ε���100g�Ȼ�����Һ�У�ǡ����ȫ��Ӧ����ˣ�ϴ�Ӹ�������������Ϊ8g�����Ȼ�������ľ̿�ڸ����»�ԭ�ؾ�ʯ����Ҫ�ɷ�BaSO4)�õ�BaS���������ټ������ᷢ�����ֽⷴӦ���õ��ġ�
��д���������ᷴӦ����һ������Ļ�ѧ����ʽ_____��
��ͨ����������������Ҫ���ֺ�����20%���ؾ�ʯ_____g��
 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�ij��Һ M ���ܺ��� NaCl��NaOH��Na2CO3��Na2SO4 �е�һ�ֻ��֣�Ϊȷ����ɷֽ���ʵ��̽����
��������Ϣ��
���� | NaCl | NaOH | Na2CO3 | Na2SO4 |
��������Һ�� pH | 7 | 13 | 11 | 7 |
��̽�����̣�
ʵ�鲽�� | ʵ������ | ʵ����� |
����һ��ȡ������Һ M ���Թ� �У��μ���ɫ��̪��Һ�� | ��ɫ��̪���ɫ | ��ͬѧ��Ϊ��Һ M ��һ������ NaOH�� ����Ըý��������жϲ�˵������_____�� |
�����������ȡ������Һ M �� �Թ��У��μ�������ϡ���ᡣ | �����ݲ��������������� ��ʹ����ʯ��ˮ����� | ��Һ M ��һ������______���ж����� ��_____���û�ѧ����ʽ��ʾ�� |
��������������ȡ������Һ M ���Թ��У��ȵμӹ�����ϡ���ᣬ�ٵμӹ�����_____��Һ�����á� | �а�ɫ���� | �ɲ������������Ŀ�֪����Һ M ��һ����_____�� |
�����ģ�ȡ�������е��ϲ��� Һ���μӹ�����_____��Һ�� | �а�ɫ���� |
��ʵ�鷴˼�����������еμӵ���Һһ��Ҫ������ԭ����_____��


 �������������� B.
�������������� B.  ʯī�ӹ��ɽ��ʯ
ʯī�ӹ��ɽ��ʯ ��¯���� D.
��¯���� D.  ����̿��������ˮ��ҵ
����̿��������ˮ��ҵ
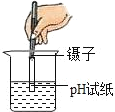 �ⶨ��ҺPH B.
�ⶨ��ҺPH B. 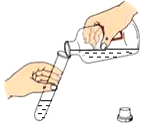 �㵹Һ��
�㵹Һ�� �������� D.
�������� D.  Ϩ��ƾ���
Ϩ��ƾ���