��Ŀ����
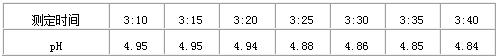
21��ijУ�������С���ͬѧ��ȡ�ս������ˮˮ������pH�Ʋ�����pH���������£�

��1����ˮ������������ʱ����ӳ���
��2��������õ�����һ���᳧�����������в���SO2����ʹ�õ�ȼ����Ҫ��ú���Է�����ɸõ����������Ҫԭ����

��1����ˮ������������ʱ����ӳ���
��ǿ
�����ǿ���������䡱������������2��������õ�����һ���᳧�����������в���SO2����ʹ�õ�ȼ����Ҫ��ú���Է�����ɸõ����������Ҫԭ����
�����������������У���ˮ�ֽ�����������������꽵�䵽�����γ����꣬���������Ἣ�ױ������е������������ᣬ������ˮ��������ʱ���ӳ�������ǿ��
��������������̼����ˮ�γ�̼�ᣬph���Ե�5.6��Ҳ����˵����������ph���������Եģ����ָ꣬�������������ԣ��������������γɵ��꣮���ң�����������ױ����������ᣬ����������������ʱ����ӳ�����ǿ��
����⣺��1��������ı����֪����ˮ�����������ʱ����ӳ�����ǿ��
��2���õ�����һ���᳧�����������в���SO2���������������������У���ˮ�ֽ�����������SO2+H2O=H2SO3�������꽵�䵽���棬����������H2SO3���ױ������е������������������H2SO3+O2=2H2SO4��������ǿ�ᣬpHֵС��5.6�������ģ�
�ʴ�Ϊ����1����ǿ��
��2�������������������У���ˮ�ֽ�����������������꽵�䵽�����γ����꣬���������Ἣ�ױ������е������������ᣬ������ˮ��������ʱ���ӳ�������ǿ��
��2���õ�����һ���᳧�����������в���SO2���������������������У���ˮ�ֽ�����������SO2+H2O=H2SO3�������꽵�䵽���棬����������H2SO3���ױ������е������������������H2SO3+O2=2H2SO4��������ǿ�ᣬpHֵС��5.6�������ģ�
�ʴ�Ϊ����1����ǿ��
��2�������������������У���ˮ�ֽ�����������������꽵�䵽�����γ����꣬���������Ἣ�ױ������е������������ᣬ������ˮ��������ʱ���ӳ�������ǿ��
������ע�������PH�ı仯�������֪������ij���PH��Ӱ�죮

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ