��Ŀ����
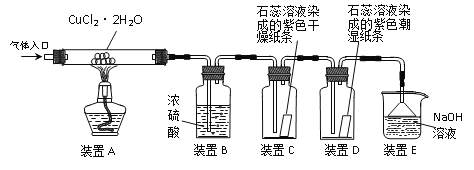
����Ŀ����ѧ��ȤС���ͬѧ����ȥijFeCl2��Һ�л��е�CuCl2���ʣ�ʵ������������£�
(1)���ڻ����Һ�м�������������ۣ�ֻ��һ����ȷѡ�ѡ����ĸ�������裬ʹ���ַ�Ӧ��
A���� B��ͭ C����
�ڹ��ˣ��õ�FeCl2��Һ�ͽ�������
��2����С��ͬѧ������̽�������ڵõ��Ľ����������Cu���������������ý��������ϴ�ӡ�����Ƶ�������Ϊ28.0g����˽������������εμ�ϡ���ᣬ�������������������ϡ������Һ��������ϵ��ͼ��ʾ������������⣺

�ټ���ý����������Cu������������������ݻ�ѧ����ʽд�������ļ��㲽�裩
�ڸ�ʵ���У���ͬѧ��Ϊ������ͨ���ⶨ��������������Cu������������ʵ��ʱ����Ҫ�ⶨ��ʵ������Ӧ��������
���𰸡� (1) ��A (2)������������Ϊx
Fe+2HCl=FeCl2+H2��
56 2
x 0.1g
56/2=x/0.1g
x=2.8g
����Ʒ��ͭ����������Ϊ(28.0g-2.8g)��28.0g��100%=90%��
�����μ����������ٲ�������ʱ�����������ϴ�ӡ������
��������(1)�ٳ�ȥijFeCl2��Һ�л��е�CuCl2���ʣ�Ӧ�ڻ����Һ�м�����������ۣ������Ȼ�ͭ��Ӧ�����Ȼ�������ͭ�����˵��Ȼ�������Һ��2)������������Ϊx
Fe+2HCl=FeCl2+H2��
56 2
x 0.1g
56/2=x/0.1g
x=2.8g
����Ʒ��ͭ����������Ϊ(28.0g-2.8g)��28.0g��100%=90%��
���������ᷴӦ����Һ��ͭ�����ᷴӦ�����μ����������ٲ�������ʱ�����������ϴ�ӡ������Ϊ�������ͭ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�