��Ŀ����
�ں�ۡ���֮�佨����ϵ�ǻ�ѧѧϰ���ص㣮
��1����ˮ��ͭ���Ȼ������������У������ӹ��ɵ�������______��
��2���ݱ�����ijЩҩ�������Ľ����С��������꣬���ڷ��û����𡰸��ж�������Ԫ�ص������Ϣ��ͼ1��ʾ����ش𣺸�Ԫ�صĺ˵����Ϊ______����ԭ��ʧȥ3�������γɵ����ӷ���Ϊ______�������ж����еĸ���ָ______������ӡ����ʡ���Ԫ�ء�����
��3��ͼ2���ܱ���ϵ��ij��Ӧ����ʾ��ͼ����
 ���͡�
���͡� ���ֱ��ʾ��ͬԭ�ӣ���Ӧ����Ӧ����������ѡ���е�1��C���ǣ�ѡ����ţ�______��
���ֱ��ʾ��ͬԭ�ӣ���Ӧ����Ӧ����������ѡ���е�1��C���ǣ�ѡ����ţ�______��
��4���Ƚ��������ֱ仯����ˮ���ȱ��ˮ��������ˮͨ��ֽ⣮�������ӵĽǶȷ��������ֱ仯�ı���������______��
��5��ͼ3��ij��Ӧ����ʾ��ͼ����������ӱ仯�ĽǶȷ����÷�Ӧ��ʵ�ʣ�
��6����������Ϊ20%������������Һ40g��һ��������ϡ����ǡ����ȫ��Ӧ���������������Ƶ������Ƕ��٣�
���𰸡���������1���������ʵĹ��ɷ������
��2������ͼʾ�й��ڸ�Ԫ�ص���Ϣ��֪���ĺ˵�������������ӵ���д������д��������λ����ɵĸ����ӣ����ж���ָ�����к��и�Ԫ�ص��µ��ж�����
��3�����������غ㶨�ɵ��۽��ͣ���Ӧǰ��ԭ�ӵ��������Ŀ������з�����
��4�����ݷ����������仯�ͻ�ѧ�仯�еı仯���з������жϣ�
��5����������������������Һ��Ӧ���۹���ʾ��ͼ���ӹ��ɴ������ӵĽǶȷ����÷�Ӧ������к͵�ʵ�ʣ�
��6���������ʵ�����=��Һ������×���ʵ�������������μӷ�Ӧ���������Ƶ����������ݷ���ʽ�������������Ƶ�������
����⣺��1��ˮ��ˮ���ӹ��ɣ�������ԭ�ӹ��ɣ��Ȼ������������Ӻ������ӹ��ɵĻ����
��2������ͼʾ�й��ڸ�Ԫ�ص���Ϣ��֪����ԭ��������24������������24���˵����Ҳ��24����ԭ��ʧȥ3�������γɵ����Ӵ���������λ������ɣ����ӷ���ΪCr3+���������������֪���ж���ָ�����к��и�Ԫ�ص��µ��ж��������ԡ����ж����еĸ���ָ��Ԫ�أ�
��3�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵ��������Ŀ���䣬����һ��C���Ӹ�ԭ�Ӹ���������ȣ���ѡC��
��4��ˮ���ȱ��ˮ��������ˮ����֮��ļ������ˮ���ӱ���û�з����仯�����������仯����ˮͨ��ֽ�Ϊ��ԭ�Ӻ���ԭ�ӣ�ÿ������ԭ�ӹ���һ�������ӣ�ÿ������ԭ�ӹ���һ������ӣ������������Ӿۼ���Ϊ����������������Ӿۼ���Ϊ�������ñ仯���ڻ�ѧ�仯��
��5��������������������Һ��Ӧ���۹���ʾ��ͼ��֪���÷�Ӧ������к͵�ʵ��Ϊ�������Ӻ����������ӽ������ˮ���ӣ�
��6���μӷ�Ӧ���������Ƶ������ǣ�40g×20%=8g
�����������Ƶ�������x
2NaOH+H2SO4�TNa2SO4+2H2O
80 142
8g x
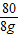 =
=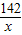
x=14.2g
�����������Ƶ�������14.2g��
�ʴ�Ϊ����1���Ȼ��ƣ�
��2��24��Cr3+��Ԫ�أ�
��3��C��
��4������ˮ����û�иı䣬���Ӽ�������ˮ���ӱ�Ϊ����Ӻ������ӣ�
��5��H+��OH-���������H2O���ӣ�
��6��14.2g��
���������⿼���֪ʶ˼ά��ȴ�����㣬�������ʵ���ɡ����ɡ�Ԫ�����ڱ���Ԫ������������ῴ��ͼ�����⻯ѧ��Ӧ��ʵ�ʣ�����ʽ�ļ����֪ʶ�����ܽ������ϸ�ķ���������ȷ���
��2������ͼʾ�й��ڸ�Ԫ�ص���Ϣ��֪���ĺ˵�������������ӵ���д������д��������λ����ɵĸ����ӣ����ж���ָ�����к��и�Ԫ�ص��µ��ж�����
��3�����������غ㶨�ɵ��۽��ͣ���Ӧǰ��ԭ�ӵ��������Ŀ������з�����
��4�����ݷ����������仯�ͻ�ѧ�仯�еı仯���з������жϣ�
��5����������������������Һ��Ӧ���۹���ʾ��ͼ���ӹ��ɴ������ӵĽǶȷ����÷�Ӧ������к͵�ʵ�ʣ�
��6���������ʵ�����=��Һ������×���ʵ�������������μӷ�Ӧ���������Ƶ����������ݷ���ʽ�������������Ƶ�������
����⣺��1��ˮ��ˮ���ӹ��ɣ�������ԭ�ӹ��ɣ��Ȼ������������Ӻ������ӹ��ɵĻ����
��2������ͼʾ�й��ڸ�Ԫ�ص���Ϣ��֪����ԭ��������24������������24���˵����Ҳ��24����ԭ��ʧȥ3�������γɵ����Ӵ���������λ������ɣ����ӷ���ΪCr3+���������������֪���ж���ָ�����к��и�Ԫ�ص��µ��ж��������ԡ����ж����еĸ���ָ��Ԫ�أ�
��3�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵ��������Ŀ���䣬����һ��C���Ӹ�ԭ�Ӹ���������ȣ���ѡC��
��4��ˮ���ȱ��ˮ��������ˮ����֮��ļ������ˮ���ӱ���û�з����仯�����������仯����ˮͨ��ֽ�Ϊ��ԭ�Ӻ���ԭ�ӣ�ÿ������ԭ�ӹ���һ�������ӣ�ÿ������ԭ�ӹ���һ������ӣ������������Ӿۼ���Ϊ����������������Ӿۼ���Ϊ�������ñ仯���ڻ�ѧ�仯��
��5��������������������Һ��Ӧ���۹���ʾ��ͼ��֪���÷�Ӧ������к͵�ʵ��Ϊ�������Ӻ����������ӽ������ˮ���ӣ�
��6���μӷ�Ӧ���������Ƶ������ǣ�40g×20%=8g
�����������Ƶ�������x
2NaOH+H2SO4�TNa2SO4+2H2O
80 142
8g x
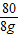 =
=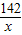
x=14.2g
�����������Ƶ�������14.2g��
�ʴ�Ϊ����1���Ȼ��ƣ�
��2��24��Cr3+��Ԫ�أ�
��3��C��
��4������ˮ����û�иı䣬���Ӽ�������ˮ���ӱ�Ϊ����Ӻ������ӣ�
��5��H+��OH-���������H2O���ӣ�
��6��14.2g��
���������⿼���֪ʶ˼ά��ȴ�����㣬�������ʵ���ɡ����ɡ�Ԫ�����ڱ���Ԫ������������ῴ��ͼ�����⻯ѧ��Ӧ��ʵ�ʣ�����ʽ�ļ����֪ʶ�����ܽ������ϸ�ķ���������ȷ���

��ϰ��ϵ�д�
�����Ŀ
��2012?��̨���ں�ۺ���֮�佨����ϵ�ǻ�ѧѧ�����е�˼ά��ʽ�����жԺ����ʵ���۽��ʹ�����ǣ�������
|
�ں�ۺ���֮�佨����ϵ�ǻ�ѧѧ�����е�˼ά��ʽ�����жԺ����ʵ���۽��Ͳ���ȷ���ǣ�������
|
�ں�ۺ���֮�佨����ϵ�ǻ�ѧѧ�����е�˼ά��ʽ�����жԺ����ʵ���۽��ʹ�����ǣ�������
|
�ں�ۺ���֮�佨����ϵ�ǻ�ѧѧ�����е�˼ά��ʽ�����жԺ����ʵ���۽��ʹ�����ǣ�������
|
| A��A | B��B | C��C | D��D |
�ں�ۺ���֮�佨����ϵ�ǻ�ѧѧ�����е�˼ά��ʽ�����жԺ����ʵ���۽��ʹ�����ǣ�������
| A��ˮ�����������������--�����ڻ�ѧ�仯�п����ٷ� | B��������ɹ�·������������--�������������˶����ʼӿ� | C��ϡ���������������--ԭ�Ӻ�����Ӵﵽ����ȶ��ṹ | D��ʯī�ͽ��ʯ�����������кܴ����--̼ԭ�ӵĽṹ��ͬ |