��Ŀ����
�ƶ������й��Ŵ���һ����Ҫ����������ɽ��ɽˮ�������Ե�������ɽˮĥ���Ƴɣ��Կڸ��ۻ�������ӯ�ڶ�����������Ҫ�ɷ������ʾ��
| ˮ��������Ҫ�ɷ� ���ʡ������������� ÿ100g���� |
| ˮ�������������������� 89.3g |
| �����ʡ����������� ������ 4.7g |
| ��֬����������������������1.3g |
| ���ࡡ�������� �������� 2.8g |
| ����B1 ���������� ������0.06mg |
| ����B2 ���������� ���� 0.03mg |
| �ơ��������������������� 0.24g |
| ���������������� �������� 1.4g |
| �������� ���� ��������0.064g |
A��ƻ���� B���㡡C���ײˡ� D������
��2�������к��зḻ���ܴٽ����������������ҪԪ�أ���Ԫ����______ ����Ԫ�ط��ţ�
��3�����������Ĺ�������һ������¼�����
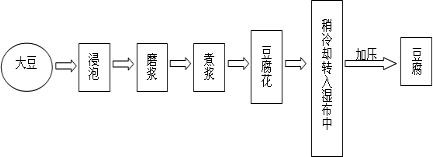
�ٽ��ݴ���ĥ�����۽������Ͷ������롡���ݵõ������� ������ȴת��ʪ���У�����������ˮ�����ѹ�õ�ʳ�ö������������������У�����ڢ�����______ �仯�����еõ���ʳ�ö���������______����������������
�⣺��1��ƻ�����ײ��к��зḻ��ά���أ������к��зḻ����֬�����к��зḻ�ĵ����ʣ�
���B��
��2����Ԫ���ܴٽ���������������ͷ�����
���Ca��
��3��ĥ�����������Ͷ�������Ĺ�����û�����������ɣ����������仯��
ʳ�ö����к���ˮ�����ࡢ�����ʡ���֬�����εȶ������ʣ����ڻ���
�������������
�������������ǹ�������ϸ���Ļ������ʣ���Щʳ���к��зḻ�ĵ����ʣ������仯��û�����������ɣ��������ʵ���ɿ����жϻ����ʹ����
�������ж�һ���仯�������仯���ǻ�ѧ�仯ʱ�����ǿ��Ƿ������������ɣ���������������ɣ����ڻ�ѧ�仯�����û�����������ɣ����������仯��
���B��
��2����Ԫ���ܴٽ���������������ͷ�����
���Ca��
��3��ĥ�����������Ͷ�������Ĺ�����û�����������ɣ����������仯��
ʳ�ö����к���ˮ�����ࡢ�����ʡ���֬�����εȶ������ʣ����ڻ���
�������������
�������������ǹ�������ϸ���Ļ������ʣ���Щʳ���к��зḻ�ĵ����ʣ������仯��û�����������ɣ��������ʵ���ɿ����жϻ����ʹ����
�������ж�һ���仯�������仯���ǻ�ѧ�仯ʱ�����ǿ��Ƿ������������ɣ���������������ɣ����ڻ�ѧ�仯�����û�����������ɣ����������仯��

��ϰ��ϵ�д�
�����Ŀ



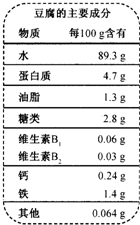 ��2008?��������ģ���ƶ������й��Ŵ���һ����Ҫ�����������ȫ���ѳ�Ϊ���ܻ�ӭ��ʳƷ������Ҫ�ɷ���ͼ��ʾ��
��2008?��������ģ���ƶ������й��Ŵ���һ����Ҫ�����������ȫ���ѳ�Ϊ���ܻ�ӭ��ʳƷ������Ҫ�ɷ���ͼ��ʾ��