��Ŀ����
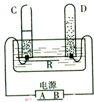 ��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش���������
��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش�����������1���ж����ӵ�Դ������������ͼ��A���ʾ
��
��
������2��ͨ��һ��ʱ����Թ�D�����ռ�������Ϊ
������O2��
������O2��
�����֤�������壨��ֻд���������ô����ǵ�ľ�������Թܿڣ�ľ����ȼ
�ô����ǵ�ľ�������Թܿڣ�ľ����ȼ
����3���÷�Ӧ�ı���ʽΪ
H2O
H2+O2
| ͨ�� |
H2O
H2+O2
��| ͨ�� |
��4��ʵ�����У����������� �ڹ��� �۾��ó��� ������Ⱦ���ˮ�IJ����У��ɽ���ˮ��Ӳ�ȵ���
��
��
������ţ�����5����Ϊ��֤��������õ���ˮ��Ӳˮ������ˮ������Ϊ������
����ˮ
����ˮ
�����飮���������ݵ��ˮʱ���������⣬�����һ���Ľ��ۺͷֽⷴӦ�ĸ�����з������ˮ���й����⣬���ݽ���Ӳ�ȵķ����ͷ���ˮ����Ӳˮ������ͬ�ж���Ӳˮ�ļ������������⣻
����⣺��1���ݵ��ˮʱ���������⣬�����һ���Ľ��ۣ���֪��A�ǵ�ظ�����
��2�����ڵ��ˮ���ɵ��������������������2��1�������Թ�D�����ռ������壨�٣�Ϊ���������������ô����ǵ�ľ�����飬�����Ǹ�ȼ��
��3���÷�Ӧ�IJ������������������ʸ÷�Ӧ�ı���ʽΪH2O
H2+O2��
��4�����ó����ܳ�ȥ�������ɳ�������̶���ͣ����������ܽ�С������ۼ��ɴ�Ŀ��������˿ɽ������ԵĹ��嶼�����������ܽ������Ե����ʶ������������̶���ߣ������Խ���ˮ��Ӳ�ȣ�
��5������ˮ��Ӳˮ��ĭ���٣�����ˮ��ĭ�϶࣬���Կ������÷���ˮ������Ӳˮ��
�ʴ�Ϊ����1����������2��������O2�����ô����ǵ�ľ�������Թܿڣ�ľ����ȼ����3��H2O
H2+O2����4���ܣ���5������ˮ��
��2�����ڵ��ˮ���ɵ��������������������2��1�������Թ�D�����ռ������壨�٣�Ϊ���������������ô����ǵ�ľ�����飬�����Ǹ�ȼ��
��3���÷�Ӧ�IJ������������������ʸ÷�Ӧ�ı���ʽΪH2O
| ͨ�� |
��4�����ó����ܳ�ȥ�������ɳ�������̶���ͣ����������ܽ�С������ۼ��ɴ�Ŀ��������˿ɽ������ԵĹ��嶼�����������ܽ������Ե����ʶ������������̶���ߣ������Խ���ˮ��Ӳ�ȣ�
��5������ˮ��Ӳˮ��ĭ���٣�����ˮ��ĭ�϶࣬���Կ������÷���ˮ������Ӳˮ��
�ʴ�Ϊ����1����������2��������O2�����ô����ǵ�ľ�������Թܿڣ�ľ����ȼ����3��H2O
| ͨ�� |
�����������Ƕ�ˮ���й�����Ŀ��飬����Ĺؼ��Ƕ�ˮ�ľ����Լ����ˮ���й�֪ʶ�����������գ�

��ϰ��ϵ�д�
�����Ŀ
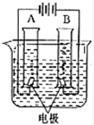 27����ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش��������⣺
27����ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش��������⣺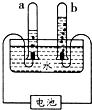 ��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã�����˵����ȷ���ǣ�������
��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã�����˵����ȷ���ǣ�������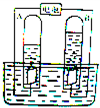 ��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã�����д���пհף�
��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã�����д���пհף�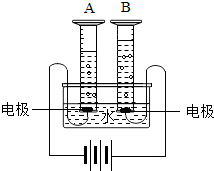 ��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش��������⣺
��ͼ��ʾͨ��ֽ�ˮ�ļ���װ�ã��ش��������⣺