��Ŀ����
��2012?�Ƹԣ���1����C��H��O��N��S��5��Ԫ����ѡ��ǡ����Ԫ�أ������ӷ��Ż�ѧʽ��գ����ж��ֵ�ֻ��Ҫд��һ�ּ��ɣ���
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ж��ֵ�ֻ��Ҫд��һ�ּ��ɣ���
| �����к������ĵ��� | 2����������� | ��Է�������Ϊ34�Ļ����� | ������Ԫ����ɵĵ��� |
| ��ʵ�����ø������������ | |
| ���Ҵ��ڿ�������ȫȼ�� | |
| ���е���ͭ�μӵ��û���Ӧ | |
| ���г������ɵĸ��ֽⷴӦ |
���������⿼�黯ѧ��������弰��д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӻ��ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ�Լ���ѧ����ʽ����������ȷ�Ľ�������Ŀ��
����⣺��1�������к����������嵥���ǵ������仯ѧʽ��N2��
���ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������������ӱ�ʾΪ2SO42-��
��������Ԫ�ؿ���ɵ���Է�������Ϊ34�Ļ�����Ϊ���⣬�仯ѧʽΪ��H2S��
̼������dz����ĵ��ʣ���笠����Ӻ�̼��������ӹ��ɣ��仯ѧʽΪ��NH4HCO3��
��2���ٸ�������ڼ��ȵ������·�Ӧ��������ء��������̡���������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4
K2MnO4+MnO2+O2����
���Ҵ��ڵ�ȼ����������������Ӧ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
��ͭ�ܽ��������е����û���������Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
�����������Ȼ�����Ӧ�������ᱵ�������Ȼ��ƣ����ڸ��ֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ��Na2SO4+BaCl2�TBaSO4��+2NaCl��
�ʴ�Ϊ����1��N2��2SO42-��H2O2��H2S�� NH4HCO3��NH4��2SO4��CO��NH2��2����NH4��2CO3�ȡ���
��2����2KMnO4
K2MnO4+MnO2+O2����
��C2H5OH+3O2
2CO2+3H2O��
��Cu+2AgNO3�TCu��NO3��2+2Ag��
��Na2SO4+BaCl2�TBaSO4��+2NaCl��
���ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������������ӱ�ʾΪ2SO42-��
��������Ԫ�ؿ���ɵ���Է�������Ϊ34�Ļ�����Ϊ���⣬�仯ѧʽΪ��H2S��
̼������dz����ĵ��ʣ���笠����Ӻ�̼��������ӹ��ɣ��仯ѧʽΪ��NH4HCO3��
��2���ٸ�������ڼ��ȵ������·�Ӧ��������ء��������̡���������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4
| ||
���Ҵ��ڵ�ȼ����������������Ӧ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
| ||
��ͭ�ܽ��������е����û���������Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3�TCu��NO3��2+2Ag��
�����������Ȼ�����Ӧ�������ᱵ�������Ȼ��ƣ����ڸ��ֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ��Na2SO4+BaCl2�TBaSO4��+2NaCl��
�ʴ�Ϊ����1��N2��2SO42-��H2O2��H2S�� NH4HCO3��NH4��2SO4��CO��NH2��2����NH4��2CO3�ȡ���
��2����2KMnO4
| ||
��C2H5OH+3O2
| ||
��Cu+2AgNO3�TCu��NO3��2+2Ag��
��Na2SO4+BaCl2�TBaSO4��+2NaCl��
������������Ҫ�����˻�ѧʽ����д�����塢�����ijɷּ����ɷֵ�����������ؿ��е�Ԫ�طֲ��뺬��

��ϰ��ϵ�д�
�����Ŀ
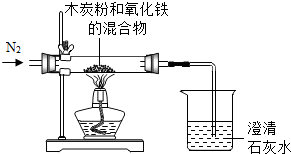 ��2012?�Ƹ�ģ�⣩��֪ľ̿�ۣ�����ľ̿���в����������ʣ���Fe2O3���ڼ��������·�����ѧ��Ӧ����ȤС��ͬѧ�Ը�ʵ���������̽����
��2012?�Ƹ�ģ�⣩��֪ľ̿�ۣ�����ľ̿���в����������ʣ���Fe2O3���ڼ��������·�����ѧ��Ӧ����ȤС��ͬѧ�Ը�ʵ���������̽����
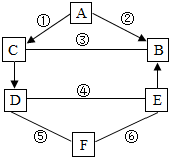 ��2012?�Ƹ�ģ�⣩A��B��C��D��E��F���dz��л�ѧ���������ʣ�����B��ֲ����й�����õ�һ����Ҫԭ�ϡ�C�ǽ������������֮��Ĺ�ϵ����ͼ��ʾ����ͼ�С�һ����ʾ���˵������ܷ�����ѧ��Ӧ����������ʾ���ʼ����ת����ϵ���йط�Ӧ������������������ȥ����
��2012?�Ƹ�ģ�⣩A��B��C��D��E��F���dz��л�ѧ���������ʣ�����B��ֲ����й�����õ�һ����Ҫԭ�ϡ�C�ǽ������������֮��Ĺ�ϵ����ͼ��ʾ����ͼ�С�һ����ʾ���˵������ܷ�����ѧ��Ӧ����������ʾ���ʼ����ת����ϵ���йط�Ӧ������������������ȥ����