��Ŀ����
����Ŀ��ij�������ʿ��ܺ���Na2SO4��Na2CO3��CaCl2��CuSO4ؼKCl��KOH�е��ֻ��֡�Ϊ̽�������,���̺������¼����:
�����ϡ�CaCl2��BaCl2��KCl������Һ��������
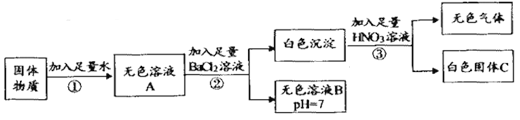
(1)���ݲ���ٵ�ʵ������ó��ù���������һ��______(ѡ��С���û��")CuSO4������ɫ��ҺB��pH=7,�ɵó��ù���������һ������______ (�ѧʽ,��ͬ)��
(2)������з����Ļ�ѧ����ʽΪ______ (д��һ������)��
(3)���ݲ���ڢ۵�����ɵó�����������һ������______���ɴ˻������ƶϳ��ù���������CaC2��______ (ѡ�һ���С�����һ��û��"���������С�)��
(4)ȡͼ����ɫ��ҺB,���εμ�AgNO3��ϡHNO3,______(ѡ��ܡ����ܡ�)����ԭ�������Ƿ���KC1,������___��
(5)������ʵ������м�����Һ���������������������ϵ��ͼ��ʾ����b��ʱ�����Ļ�ѧʽΪ______�ڲ���۹�����,�������������Ϊ0.44g,��m�����ֵ��______��
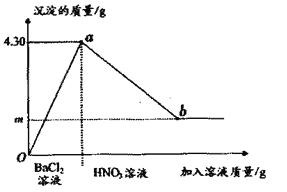
���𰸡� û�� KOH Na2SO4 + BaCl2 = BaSO4 �� +2NaCl �� Na2CO3 + BaCl2 = BaCO3 �� +2NaCl ���ɣ� Na2SO4��Na2CO3 ��©�������֣� һ��û�� ���� ������м���BaCl2����Cl- BaSO4 2.33
��������������ѧ֪ʶ��������Ϣ֪������ˮ����ɫ��Һ����û������ͭ��̼���ƻ��Ȼ��ơ������������Ȼ�����Һ���а�ɫ����������һ����̼���ƻ������ƣ���Һ�����ԣ�һ��û���������ء��������������ᣬ����ɫ����Ͱ�ɫ�������ɣ�һ����̼���ƺ������ơ���1)���ݲ���ٵ�ʵ������ó��ù���������һ��û��CuSO4������ɫ��ҺB��pH=7,�ɵó��ù���������һ������KOH��(2)������з����Ļ�ѧ����ʽΪNa2SO4 + BaCl2 = BaSO4 �� +2NaCl��(3)���ݲ���ڢ۵�����ɵó�����������һ������Na2SO4��Na2CO3�����ɴ˻������ƶϳ��ù���������CaCl2��һ��û��. (4)ȡͼ����ɫ��ҺB,���εμ�AgNO3��ϡHNO3, ���ܼ���ԭ�������Ƿ���KC1,�����Dz�����м���BaCl2����Cl-.(5)������ʵ������м�����Һ���������������������ϵ��ͼ��ʾ����b��ʱ�����Ļ�ѧʽΪBaSO4���ڲ���۹�����,�������������Ϊ0.44g,��m�����ֵ�ǡ�BaCO3��CO2 �� ![]() ��
��![]() ��x��2.33g.
��x��2.33g.
�㾦��������Ҫ�������ʳɷֵ��ƶϡ�

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�