��Ŀ����
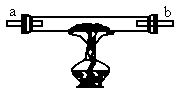
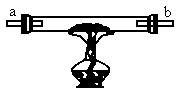
�����ֺ�ɫ��ĩA��B��������ɫ����C��D����ͼ6-6��װ��ͼ��ʾ��װ����������ʵ�飺

ͼ6-6
��һ���������ȹ���ʢ�еĺ�ɫ��ĩAʱ����a�˵���ͨ������D�������b�˲�������C�����ڵĺ�ɫ��ĩ��Ϊ����ɫ��
�ڶ����������ȹ���ʢ�ĺ�ɫ��ĩBʱ���ɵ���a��ͨ������C�������b�˲�������D������D����ȼ�գ�
������������a�˶��ϣ����ȹ������ֺ�ɫ����Ļ��������b��Ҳ��������C��
��������ʵ����ʵ����������⣺
��1��д�������ʵ����ƣ�
A________��B________��C________��D________��
��2��д��������Ӧ�Ļ�ѧ����ʽ��
��__________ ______����________________����________________��
______����________________����________________��
��𣺣�1��A��B���Ǻ�ɫ��ĩ����һ��A�ܱ�D��ԭ������ɫͭ��˵��A������ͭ���ڶ���B�ܰ�C��ԭ�ɿ�ȼ������D��������A��B��Ϲ�������C��˵��B��̼��
C��D ���ֶ�����ɫ���壬C��D֮����Ի���ת����D�л�ԭ�Լ���ȼ�ԣ�˵��D��һ����̼��C�Ƕ�����̼��
���ֶ�����ɫ���壬C��D֮����Ի���ת����D�л�ԭ�Լ���ȼ�ԣ�˵��D��һ����̼��C�Ƕ�����̼��
��2����ѧ����ʽ��
��CuO+CO Cu+CO2 ��CO2+C
Cu+CO2 ��CO2+C 2CO ��2CuO+C
2CO ��2CuO+C 2Cu+CO2��
2Cu+CO2��

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
�����Ŀ
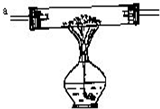 �����ֺ�ɫ��ĩA��B��������ɫ����C��D���������װ��ͼ��ʾ��װ����������ʵ�飺
�����ֺ�ɫ��ĩA��B��������ɫ����C��D���������װ��ͼ��ʾ��װ����������ʵ�飺