��Ŀ����
��ѧΪ�������Ŀɳ�����չ�����˾��ף�
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ��������������______��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ�
��2��������δ����������Դ��������Ϊ������������Ҫԭ�ϣ�Ҳ���������칤ҵ�ĸ���ȼ�ϣ���ȼ�յĻ�ѧ����ʽ��______��
��3������������������Ч������Դ�����ͳ��Դ��������δ�����������Դ������Ϊ����������ⷽ����______������ţ���
A�����ˮ��2H2O�T2H2��+O2��
B����̿��ˮ������Ӧ��C+H2O
H2+CO
C��̫����ֽ�ˮ��2H2O
2H2��+O2��
D����Ȼ����ˮ������Ӧ��CH4+H2O
CO+3H2��
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ��������������______��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ�
��2��������δ����������Դ��������Ϊ������������Ҫԭ�ϣ�Ҳ���������칤ҵ�ĸ���ȼ�ϣ���ȼ�յĻ�ѧ����ʽ��______��
��3������������������Ч������Դ�����ͳ��Դ��������δ�����������Դ������Ϊ����������ⷽ����______������ţ���
A�����ˮ��2H2O�T2H2��+O2��
B����̿��ˮ������Ӧ��C+H2O
| ||
C��̫����ֽ�ˮ��2H2O
| ||
| ̫���� |
D����Ȼ����ˮ������Ӧ��CH4+H2O
| ||
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ��������������ú��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ�
��2������ȼ��������ˮ����Ӧ�ķ���ʽ�ǣ�2H2+O2
2H2O��
��3������ȡ�����ĸ���ѧ��Ӧ����ʽ��֪��AC����������������������BD����������һ����̼���������ɼ��������ȡ�����ķ�����AC���ӷ�Ӧ������֪��C������̫���⣬��Լ��Դ���������������C��
�ʴ�Ϊ����1��ú����2��2H2+O2
2H2O����3��C��
��2������ȼ��������ˮ����Ӧ�ķ���ʽ�ǣ�2H2+O2
| ||
��3������ȡ�����ĸ���ѧ��Ӧ����ʽ��֪��AC����������������������BD����������һ����̼���������ɼ��������ȡ�����ķ�����AC���ӷ�Ӧ������֪��C������̫���⣬��Լ��Դ���������������C��
�ʴ�Ϊ����1��ú����2��2H2+O2
| ||

��ϰ��ϵ�д�
�����Ŀ
 H2+CO
H2+CO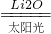 2H2��+O2��
2H2��+O2��