��Ŀ����
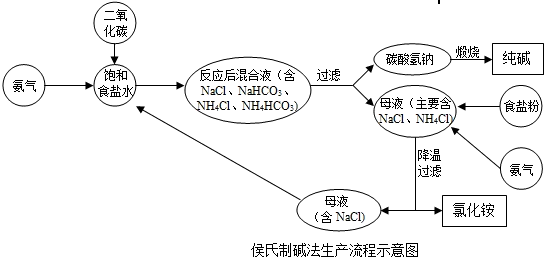
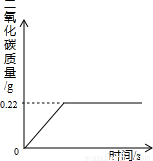
 �ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
�ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ�������������⣺
��1����Ӧ����������CO2������
��2��100g�������NaHCO3��������
��3���������Na2CO3������������
����������ͼ���֪��Ӧ�����Ķ�����̼������Ϊ0.22�ˣ���Ҫ���NaHCO3��������Na2CO3��������������ؼ�����Ҫ֪��ֻ��NaHCO3����ʱ����ֶ�����̼���壬�������ǾͿ��Ը��ݶ�����̼������ͨ������ʽ���Ƶ���NaHCO3���������Ӷ����������Na2CO3������������
����⣺��1����ͼ���֪�����ɶ�����̼������Ϊ0.22g
��2����������NaHCO3������Ϊx
2NaHCO3
Na2CO3+H2O+CO2��
168 44
x 0.22g
=
x=0.84g
��3���������Na2CO3������������
��100%=99.16%
�𣺣�2��100g�������NaHCO3������Ϊ0.84g
��3���������Na2CO3����������Ϊ99.16%
��2����������NaHCO3������Ϊx
2NaHCO3
| ||
168 44
x 0.22g
| 168 |
| 44 |
| x |
| 0.22g |
x=0.84g
��3���������Na2CO3������������
| 100g-0.84g |
| 100g |
�𣺣�2��100g�������NaHCO3������Ϊ0.84g
��3���������Na2CO3����������Ϊ99.16%
�����������Ĺؼ���Ҫѧ���ͼ��Ū��ͼ���۵������ĺ��壬����ͼ�Ż����������⣮

��ϰ��ϵ�д�
�����Ŀ