��Ŀ����
����Խ����Ŀ�����������ѭ���ģ�
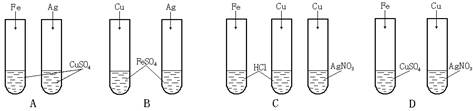
��1����ͭ�����������ʹ�õĽ�����Ʒ������ʱ�ڣ�������Ҫ���á�ʪ��ұ�𡱣��罫����������ͭ��Һ�У��÷�Ӧ�Ļ�ѧ����ʽΪ��_________����
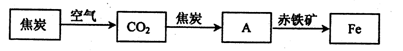
��2�����ż����IJ��Ͻ��������������û�ԭ���ӽ������������н��仹ԭ���������磬��¯�������漰������ת���������£�
���У�����AΪ��_____�����ѧʽ����A��������е���������Ӧ�Ļ�ѧ����ʽΪ��_________����
��3��ÿ����Ϊ��ʴ���������⣩�����ϵĽ����൱���������20%��40%�����Dz��ö��ַ�����ֹ������ʴ�����磬����ϴ����������ɺ��ſ��Է�ֹ�������⣬��ԭ������_________����
��4�����Ľ�����Ա�����_________�����ǿ������������������Ʒȴ���бȽϺõĿ���ʴ���ܣ���ԭ������_________�����ñ�Ҫ�����ֺͻ�ѧ����ʽ˵������

��1����ͭ�����������ʹ�õĽ�����Ʒ������ʱ�ڣ�������Ҫ���á�ʪ��ұ�𡱣��罫����������ͭ��Һ�У��÷�Ӧ�Ļ�ѧ����ʽΪ��_________����
��2�����ż����IJ��Ͻ��������������û�ԭ���ӽ������������н��仹ԭ���������磬��¯�������漰������ת���������£�

���У�����AΪ��_____�����ѧʽ����A��������е���������Ӧ�Ļ�ѧ����ʽΪ��_________����
��3��ÿ����Ϊ��ʴ���������⣩�����ϵĽ����൱���������20%��40%�����Dz��ö��ַ�����ֹ������ʴ�����磬����ϴ����������ɺ��ſ��Է�ֹ�������⣬��ԭ������_________����
��4�����Ľ�����Ա�����_________�����ǿ������������������Ʒȴ���бȽϺõĿ���ʴ���ܣ���ԭ������_________�����ñ�Ҫ�����ֺͻ�ѧ����ʽ˵������

��1��Fe + CuSO4 = FeSO4 + Cu
��2��CO ��Fe2O3 + 3CO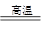 2Fe + 3CO2
2Fe + 3CO2
��3����ֹ����ˮ�Ӵ����������ɣ�
��4��ǿ ����������е�������Ӧ������һ�����ܵ���������Ĥ
��2��CO ��Fe2O3 + 3CO
 2Fe + 3CO2
2Fe + 3CO2��3����ֹ����ˮ�Ӵ����������ɣ�
��4��ǿ ����������е�������Ӧ������һ�����ܵ���������Ĥ
�����������1���ڽ������˳���У���λ��ͭ��ǰ�棬�ܰ�����ͭ��Һ�е�ͭ�û�������
��2��������ԭ��������һ����̼��ԭ�������������Ͷ�����̼��
��3�����������������������ˮͬʱ�Ӵ���ΪΪ��ֹ�����⣬���ƻ����������������Ϳ����ȣ�
��4����������е�������Ӧ������һ�����ܵ���������Ĥ������ֹ���Ľ�һ��������

��ϰ��ϵ�д�
 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ