��Ŀ����
����Ŀ���������������������������Ź㷺��Ӧ�ã�
(1)��ͭ˿��������������ͭ��_______�ԡ�
(2)������ԭ��������һ����̼����������Ӧ��ʵ��װ�ü�ͼ�ף�

�ٶ�ʵ����з������ش�ʵ����Ӳ�ʲ����������Ӧ��������________________������A��������Ӧ�Ļ�ѧ����ʽ��____________��
��ʵ��Ҫ��װ��ĩ��a��ȼ��һյ�ƾ��ƣ���������____________��
(3)��ҵ�����У��и�����ʱ������ͭ��Һ�������ϻ��߿����º�ɫ��ӡ�����йط�Ӧ�Ļ�ѧ����ʽΪ____________________________������____��Ӧ��
(4)Ҫ�Ƚ���������ͭ�Ľ������ǿ����ѡ���ҩƷ�����������⣬����Ҫ__��Һ��
(5)ij�о���ѧϰС��Ϊ�˲ⶨ��ͭ(ͭ��п�Ͻ�)����ɣ��õ�����ƽ�ֱ�Ƶ���ƿ����������Ϊ44.1g����ȡ��ͭ��Ʒ20.0g������ƿ�м������Ʒ������ϡ�����ƿ������������ͼ����ʾ����������ƽ���������ݻ��ͼ����
����ʵ�鼰ʵ�����ݻش𣺢ٸ÷�Ӧ��������������Ϊ_________________��
����Ʒ��ͭ������������__________��(д���������)
���𰸡� ������ ��ɫ�����ɺ�ɫ CO2+Ca(OH)2==CaCO3��+H2O ��ȥCO����ֹCO��Ⱦ���� Fe + CuSO4 == Cu + FeSO4 �û���Ӧ ����ͭ��CuSO4 0.4g 35%
����������1��ͭ���е����ԣ����������ߣ�
��2����Ӳ�ʲ���������һ����̼�������������ķ�Ӧ����Ӧ����������������ԭΪ������ɫ�ɺ�ɫ��Ϊ��ɫ��A�Թ��������ɵĶ�����̼�������������Ʒ�Ӧ����̼��ƺ�ˮ����Ӧ�ķ���ʽΪ��CO2+Ca(OH)2==CaCO3��+H2O ��
�ڸ÷�Ӧ����һ����̼�μӣ���Ӧ���β���л���һ����̼������Ⱦ���������þƾ��Ƶ�ȼһ����̼����ֹ��Ⱦ������
��3����������ͭ��Ӧ����ͭ��������������Ӧ����ʽΪFe + CuSO4 == Cu + FeSO4���÷�Ӧ��һ�ֵ�����һ�ֻ����ﷴӦ������һ�ֵ��ʺ���һ�ֻ�������û���Ӧ��
��4�������Խ�����ͭ�е�ͭ�û��������������ܰ�����ͭ�е�ͭ�û����������Եó���![]() ͭ
ͭ![]() ������ѡ����ͭ��Һ��
������ѡ����ͭ��Һ��
��5����������ͼ��֪����Ӧǰ��������Ϊ212.5g����Ӧ��������Ϊ212.1g������������ ������Ϊ212.5g-212.1g=0.4g��
�����ͭ��Ʒ��п������Ϊx
Zn+ H2SO4 == ZnSO4 + H2��
65 2
X 0.4g
![]() =
=![]() x=13g
x=13g
��20g��Ʒ��ͭ������Ϊ20g-13g=7g
��ͭ��Ʒ��ͭ����������Ϊ�� ![]() 35%
35%
�𣺻�ͭ��Ʒ��ͭ����������Ϊ35%��

����Ŀ����������ʵ�Ľ�����ȷ���ǣ���
��ʵ | ���� | |
A | ���������и���� | �����п�ȼ�� |
B | ϴ�ྫ�����ڳ����� | ϴ�ྫ���ܽ����� |
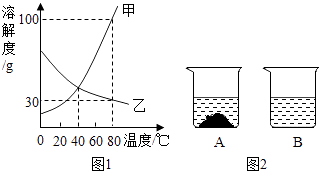
C | ������������ | �¶����ߣ�������������� �� |
D | ����˿ȼ��ʵ��ʱ����ƿ�ײ�Ԥ��Ҫװ������ ˮ | ��ֹƿ��ը�� |
A.AB.BC.CD.D