��Ŀ����
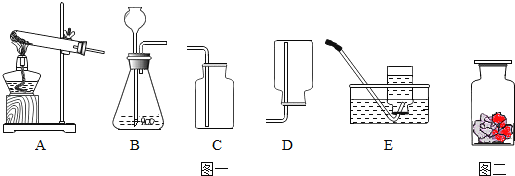
����Ŀ��С���о����ᡢ���������������ʵĻ�ѧ���ʣ�������ͼ��ʾ8��ʵ�飮
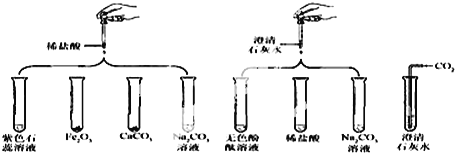
��1��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թܱ�� �����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թܱ�� �������м��������� ����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е������� ��
��3��ʵ����Թܱ�� ���ײ��а�ɫ���壮
��4��ʵ�����Թܱ�� �����ð���ݵ�����
��5���ֱ�д���Թܢ��ڵķ�Ӧ��ѧ����ʽ ��
���𰸡���1���ڣ�Fe2O3+6HCl�T2FeCl3+3H2O
��2���������ᣬ��ɫ��̪��Һ��3���ߢ�
��4���ۢ���5��2HCl+Ca��OH��2�TCaCl2+2H2O��
����������1��ʵ���ij�Թ���Ϊ��ɫ��Һ��˵������������ϡ���ᷴӦ�������Ȼ�����ˮ������Թ��з�����Ӧ�Ļ�ѧ����ʽΪFe2O3+6HCl�T2FeCl3+3H2O������ڣ�Fe2O3+6HCl�T2FeCl3+3H2O��
��2��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м���������ij��Һ���Ϊ��ɫ��˵��ԭ�Թ���ʢ�е��Ƿ�̪��Һ����������������ᣬ����ݣ����ᣬ��ɫ��̪��Һ��
��3��ʵ����Թܢߢ��еõ���ɫ����������ߢࣻ
��4��̼��ƺ�̼��������ϡ���ᷴӦ�������壬����ۢܣ�
��5���Թܢ����������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�����2HCl+Ca��OH��2�TCaCl2+2H2O��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�