��Ŀ����
����Ŀ����λ��ѧ�Ҽҿ��������߷ֱ��ʵ�ӫ������������ӫ�⵰���ӵ�ӫ�⣬�����Խ���������չ�����׳߶ȣ�����ӫ����������ȷ��̽�۵��������硣
��1��ӫ�⵰����һ����Է���������С���ܷ��⵰���ʣ�������ǻ�ϸ�����о��ض��ķ�Ӧ����ȩ��ʹӫ�⵰��ʧȥԭ�е��������ԣ�������______�����������ѧ�����仯�����ھƾ���������ӫ�⵰�ף������_________����ζ��
��2��ӫ�⵰����ͨ�������������䷢�⣬��ӫ�⵰���ӵķ����ǽ���ѧ��ת��Ϊ____�ܡ�
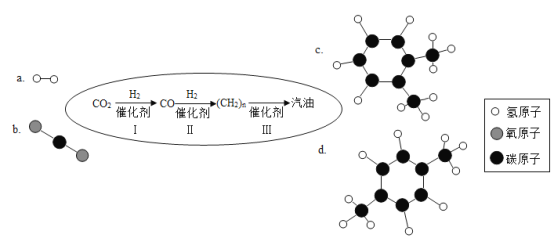
��3���ʰ��������ӫ�⵰��һ�ֳɷ֣��ʰ���ķ��ӽṹģ����ͼ��ʾ����ʰ�������______����л��������������������̼����Ԫ��֮���������Ϊ____��̼Ԫ�ص���������Ϊ_____��

��4���������еĸʰ����������ڱ��������������������������衣�䷴Ӧԭ���û�ѧ����ʽ��ʾΪ��2C2H5NO2+3O2= CO(NH2)2+3R+3CO2������������֮һR�Ļ�ѧʽΪ___________��
��5�����׳߶ȵ�ӫ������������ȫ����ر��㷺ʹ�á�����ͻ���Լ�����Ӧ�õ���Щ���棿��Ҫ���������㣩____________��
���𰸡���ѧ ��һ���ս���ë �� �л� 24:5 32% H2O �۲�ϸ���е������ӵ��˶����ڷ��Ӳ���Ϲ۲���ϸ��ͻ���Ĵ���۲��뼲����صĵ����ʾۺ����������������ٵ����ʵķ��ѵ�
��������
��1��ӫ�⵰����һ����Է���������С���ܷ��⵰���ʣ�������ǻ�ϸ�����о��ض��ķ�Ӧ����ȩ��ʹӫ�⵰��ʧȥԭ�е��������ԣ����������ʣ������˻�ѧ�仯�����ھƾ���������ӫ�⵰�ף��������һ���ս���ë����ζ��ȼ�յ����ʵ�ζ����
��2��ӫ�⵰����ͨ�������������䷢�⣬��ӫ�⵰���ӵķ����ǽ���ѧ��ת��Ϊ���ܡ�
��3���ʰ��������ӫ�⵰��һ�ֳɷ֣��ʰ����к���̼���⡢������Ԫ�أ���ʰ��������л����������̼����Ԫ��֮���������Ϊ��12��2������1��5��=24:5��̼Ԫ�ص���������=![]() ��
��
��4���������еĸʰ����������ڱ��������������������������衣�䷴Ӧԭ���û�ѧ����ʽ��ʾΪ��2C2H5NO2+3O2= CO(NH2)2+3R+3CO2���÷�Ӧ���������غ㶨�ɣ�������ȷ�Ӧ����6����ԭ�Ӻ�3����ԭ�ӣ�R�Ļ�ѧ������Ϊ3����R�Ļ�ѧʽΪ��H2O��
��5�����׳߶ȵ�ӫ������������ȫ����ر��㷺ʹ�á�����ͻ���Լ�����Ӧ�õ����۲�ϸ���е������ӵ��˶����ڷ��Ӳ���Ϲ۲���ϸ��ͻ���Ĵ���۲��뼲����صĵ����ʾۺ����������������ٵ����ʵķ��ѵȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�