��Ŀ����
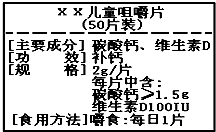
��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������⣮��1��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ______��
�������ѣ��ٸ�Ƭ�еĸ�Ԫ��ֻ������̼��ƣ�CaCO3���У�
��ֻҪд������ʽ����Ҫ��������
��2��С��ͬѧΪ�ⶨ����̼��Ƶĺ�����ע�Ƿ���ʵ��
��ȡ��10Ƭ��Ƭ����������С�ձ��У��ټ���������ϡ���ᣨHCl������÷�Ӧ�����ɶ�����̼������Ϊ4.4�ˣ�
��ͨ�������жϸø�Ƭ��̼��ƣ�CaCO3���ĺ�����ע�Ƿ���ʵ��
��̼�����ϡ���ᷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2����

���𰸡���������1��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����=ÿƬ��Ƭ�к�̼��Ƶ�����×̼����и�Ԫ�ص�����������
��2�����ݻ�ѧ����ʽ�����ɵĶ�����̼�����������ɼ����10Ƭ��Ƭ�к�̼��Ƶ�������Ȼ��ͱ�ǩ��ʾ�����ݽ��бȽϣ��Ϳ�֪���ø�Ƭ��̼��ƣ�CaCO3���ĺ�����ע�Ƿ���ʵ��
����⣺��1��̼����и�Ԫ�ص���������=1.5g× ×100%��
×100%��
�ʴ�Ϊ��1.5g× ×100%��
×100%��
��2����10Ƭ��Ƭ�к�̼��Ƶ�����Ϊx��
CaCO3+2HCl��CaCl2+H2O+CO2��
100 44
x 4.4g
��100��44=x��4.4g
��֮�ã�x=10g��
���ݱ�ǩ��ʾ��̼��ơ�1.5g����ô10Ƭ��Ƭ�к�̼��Ƶ�����ӦΪ1.5g×10=15g��10g��
�ʸø�Ƭ��̼��ƣ�CaCO3���ĺ�����ע����ʵ��
������������Ҫ����ѧ������Ԫ�ص�����������ʽ�ͻ�ѧ����ʽ���м����������
��2�����ݻ�ѧ����ʽ�����ɵĶ�����̼�����������ɼ����10Ƭ��Ƭ�к�̼��Ƶ�������Ȼ��ͱ�ǩ��ʾ�����ݽ��бȽϣ��Ϳ�֪���ø�Ƭ��̼��ƣ�CaCO3���ĺ�����ע�Ƿ���ʵ��
����⣺��1��̼����и�Ԫ�ص���������=1.5g×
 ×100%��
×100%���ʴ�Ϊ��1.5g×
 ×100%��
×100%����2����10Ƭ��Ƭ�к�̼��Ƶ�����Ϊx��
CaCO3+2HCl��CaCl2+H2O+CO2��
100 44
x 4.4g
��100��44=x��4.4g
��֮�ã�x=10g��
���ݱ�ǩ��ʾ��̼��ơ�1.5g����ô10Ƭ��Ƭ�к�̼��Ƶ�����ӦΪ1.5g×10=15g��10g��
�ʸø�Ƭ��̼��ƣ�CaCO3���ĺ�����ע����ʵ��
������������Ҫ����ѧ������Ԫ�ص�����������ʽ�ͻ�ѧ����ʽ���м����������

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
 ��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮
��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮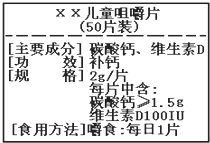 ��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮��Ҫ�ɷ�̼��ƵĻ�ѧʽ��CaCO3
��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮��Ҫ�ɷ�̼��ƵĻ�ѧʽ��CaCO3 ��ѧ�����������ߣ��������ǵ�����ϢϢ��أ�
��ѧ�����������ߣ��������ǵ�����ϢϢ��أ�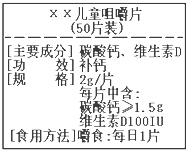 ��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������˵����ȷ���ǣ�������
��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������˵����ȷ���ǣ�������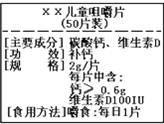 ��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮
��ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮