��Ŀ����
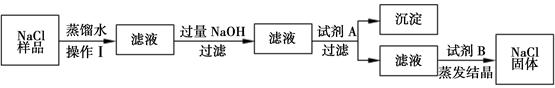
��8�֣�ijͬѧ����ʵ��ʱ��֪�Ȼ�����Ʒ�к���������ɳ���Ȼ��ƺ��Ȼ�þ������������������̳�ȥ�Ȼ�����Ʒ����ɳ���Ȼ��ƺ��Ȼ�þ��
���������ͼ�ش�
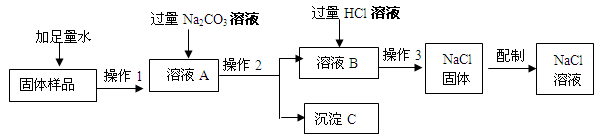
��1��������������� ���Լ�A�� ���Լ�B�� ��
��2��д��NaOH�����ʷ�Ӧ�Ļ�ѧ����ʽ ��
��3�������ᾧʱ�������������� ��
��4�����ᴿ����Ȼ��ƹ���������50g��������Ϊ6%���Ȼ�����Һ���䲽��Ϊ���ټ��㡢�ڳ������� ���� �ܽ⡢��װ���Լ�ƿ�У��Ǻ�ƿ���� ��

���������ͼ�ش�
��1��������������� ���Լ�A�� ���Լ�B�� ��
��2��д��NaOH�����ʷ�Ӧ�Ļ�ѧ����ʽ ��
��3�������ᾧʱ�������������� ��
��4�����ᴿ����Ȼ��ƹ���������50g��������Ϊ6%���Ȼ�����Һ���䲽��Ϊ���ټ��㡢�ڳ������� ���� �ܽ⡢��װ���Լ�ƿ�У��Ǻ�ƿ���� ��
��1������;Na2CO3 ; HCl��2��2NaOH+MgCl2=Mg(OH)2��+2NaCl��3�����裬��ֹ��ֲ��¶ȹ��ߣ����Һ�ηɽ���4����ȡ ���ϱ�ǩ
�����������1����Ϊ����һ��Һ���룬�ʲ������������ ���ˣ��Լ�AΪ��ȥ�����ӵ�̼������Һ���Լ�B�dz�ȥ�������������ƺ�̼���Ƶļ���ϡ�����2��NaOH�������Ȼ�þ��Ӧ�Ļ�ѧ����ʽΪ2NaOH+MgCl2=Mg(OH)2��+2NaCl����3�������ᾧʱ�������������ǽ��裬��ֹ��ֲ��¶ȹ��ߣ����Һ�ηɽ�����4�����ᴿ����Ȼ��ƹ���������50g��������Ϊ6%���Ȼ�����Һ���䲽��Ϊ���ټ��㡢�ڳ���������ȡ���� �ܽ⡢��װ���Լ�ƿ�У��Ǻ�ƿ�����ϱ�ǩ��

��ϰ��ϵ�д�
�����Ŀ