��Ŀ����
��7�֣�A��B��C��D��E��ƿ��ɫ����Һ���ֱ���ϡ���ᡢNa2CO3��Һ��MgCl2��Һ��NaNO3��Һ��NaOH��Һ�е�һ�֡��ֱ��������ʵ�飺
��A�ֱ����B��C��D��E�У���û����������
��B�ֱ����C��D��E�У�C��E�о�������ɫ������D��û����������
��C�ֱ����D��E�У�D��������ð����E��û����������
��1��A�� ��B�� ��C�� ��
��2��B����E�еĻ�ѧ����ʽ�� ��
��3��C����D�еĻ�ѧ����ʽ�� ��
��1��NaNO3��Һ��1�֣� MgCl2��Һ��1�֣� Na2CO3��Һ��1�֣�
��2��MgCl2+2NaOH===2NaCl+Mg(OH)2�� ��2�֣�
��3��Na2CO3+2HCl===2NaCl+ H2O+ CO2�� ��2�֣�
����

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
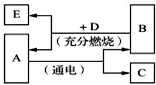 ��֪��A����Է���������С�������D������л�������A��B��C��D��E��������֮��ı仯��ϵ���ش��й����⣮
��֪��A����Է���������С�������D������л�������A��B��C��D��E��������֮��ı仯��ϵ���ش��й����⣮
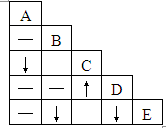 ��A��B��C��D��E��ƿʧȥ��ǩ����Һ����ƿ������Na2CO3��BaCl2��Ca��NO3��2��ϡ���ᡢAgNO3��Һ�е�һ�֣��ֽ�ƿ����Һ��ȡ���������в���������ϵ�ʵ�飬ʵ��������ͼ��˵�������С���ʾ���������ɣ�����ʾ�г������ɣ�-��ʾ��������������ܻ��������ɣ��ո��ʾʵ��û����
��A��B��C��D��E��ƿʧȥ��ǩ����Һ����ƿ������Na2CO3��BaCl2��Ca��NO3��2��ϡ���ᡢAgNO3��Һ�е�һ�֣��ֽ�ƿ����Һ��ȡ���������в���������ϵ�ʵ�飬ʵ��������ͼ��˵�������С���ʾ���������ɣ�����ʾ�г������ɣ�-��ʾ��������������ܻ��������ɣ��ո��ʾʵ��û���� ��2013?�ൺ������A��B��C��D��E�������ʣ��ֱ�Ϊ���ᡢ����ͭ���������ơ����ۺ��������е�һ�֣����У�A��Ũ��Һ����ɫҺ�壬�д̼�����ζ���ڿ����л��γɰ�����BΪ���ʣ�D�ǡ�������Һ������Ҫ�ɷ�֮һ��E����ʯ�ҵ���Ҫ�ɷ֣���ش��������⣺
��2013?�ൺ������A��B��C��D��E�������ʣ��ֱ�Ϊ���ᡢ����ͭ���������ơ����ۺ��������е�һ�֣����У�A��Ũ��Һ����ɫҺ�壬�д̼�����ζ���ڿ����л��γɰ�����BΪ���ʣ�D�ǡ�������Һ������Ҫ�ɷ�֮һ��E����ʯ�ҵ���Ҫ�ɷ֣���ش��������⣺