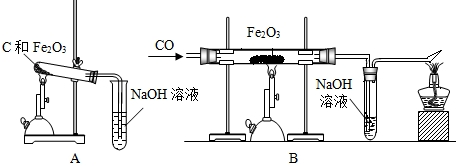
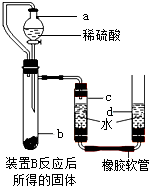
��Ŀ����
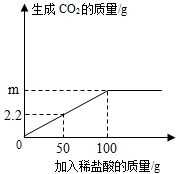 Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ�����������ȷ��ȡ12.5gʯ��ʯ��Ʒ�����������ձ��У������м�������ϡ���ᣬ�����ʲ������ᷴӦ��Ҳ������ˮ����ʵ���õ���������ͼ��ʾ����ע��CaCO3+2HCl=CaCl2+H2O+CO2����
Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ�����������ȷ��ȡ12.5gʯ��ʯ��Ʒ�����������ձ��У������м�������ϡ���ᣬ�����ʲ������ᷴӦ��Ҳ������ˮ����ʵ���õ���������ͼ��ʾ����ע��CaCO3+2HCl=CaCl2+H2O+CO2������1����ʯ��ʯ��Ʒ�����Ŀ����
ʹ̼��Ʒ�Ӧ��ȫ
ʹ̼��Ʒ�Ӧ��ȫ
����2��12.5g��Ʒ��ȫ��Ӧ���ɶ�����̼������m=
4.4
4.4
g����3�����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��������1�����ݷ�������ʼ�ĽӴ�����ִӶ��ܴٽ���Ӧ�ﷴӦ�ĸ����������
��2�����ݷ�Ӧ�������������ϵ�ж����ɶ�����̼���������ɣ�
��3�����ö�����̼��������Ϸ�Ӧ�ķ���ʽ���̼��Ƶ��������������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��2�����ݷ�Ӧ�������������ϵ�ж����ɶ�����̼���������ɣ�
��3�����ö�����̼��������Ϸ�Ӧ�ķ���ʽ���̼��Ƶ��������������ʯ��ʯ��Ʒ��̼��Ƶ�����������
����⣺��1������ʯ��ʯ��Ʒ�����̼��ƺ�����ĽӴ������Ӷ�����ʹ̼��Ʒ�Ӧ��ȫ��
��2����ͼ���֪50g�����������2.2g������̼����100g���ᷴӦ������ɶ�����̼4.4g����m=4.4g��
��3����̼��Ƶ�������x
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
=
x=10g
���ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
��100%=80%��
�ʴ�Ϊ����1��ʹ̼��Ʒ�Ӧ��ȫ����2��4.4����3����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ80%��
��2����ͼ���֪50g�����������2.2g������̼����100g���ᷴӦ������ɶ�����̼4.4g����m=4.4g��
��3����̼��Ƶ�������x
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| x |
| 44 |
| 4.4g |
x=10g
���ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ
| 10g |
| 12.5g |
�ʴ�Ϊ����1��ʹ̼��Ʒ�Ӧ��ȫ����2��4.4����3����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ80%��
������ȷ�ж�ͼ�п̶�ֵ����ȷ�������һ����Ҫϸ�ڣ������ȷ�ж����ɶ�����̼�����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ