��Ŀ����
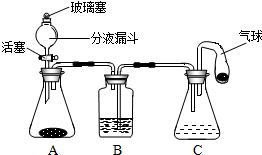
��ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�顣Aװ����װ�������ԼΪ3:1�Ŀ����Ͷ�����̼�Ļ������Bװ���� ʢ���������ۣ�Cװ����ʢ��������ϡ���ᡣ

��1���رջ����ˣ�����Aװ���н�ͷ�ι���������Ũ��������
��Һ����ƿ�У����Թ۲쵽Aװ���е������� ��Aװ�÷�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����������ˣ�һ��ʱ��رջ����ˡ�Bװ���з�����Ӧ�Ļ�ѧ![]() ����ʽΪ ��������ʵ������У����Թ۲쵽װ��B��C�е�ʵ�������� ��
����ʽΪ ��������ʵ������У����Թ۲쵽װ��B��C�е�ʵ�������� ��
��3��Cװ�õ������� ������ĸ����
a���ṩҩƷ b�������������� c������װ���ڵ�ѹǿ d����������������
��1�����������Ǵ� 2 NaOH+CO2 = Na2CO3+H2O
��2��2HCl+Fe = FeCl2+H2�� Cװ���е�ϡ��������Bװ���У�Bװ���������٣����������ݣ���Һ����ɫ���dz��ɫ��Cװ�õ�ϡ������������ð����
��3��a c b

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ
 ��ʦ����ͼ��ʾװ��Ϊͬѧ������һ����Ȥʵ�飮Aװ����ʢ�ж������̺�ɫ��ĩ��Bװ����ʢ�������ij���ʯ��ˮ��Cװ����ʢ��������ϡ���ᣬ������װ��������̼��ط�ĩ��
��ʦ����ͼ��ʾװ��Ϊͬѧ������һ����Ȥʵ�飮Aװ����ʢ�ж������̺�ɫ��ĩ��Bװ����ʢ�������ij���ʯ��ˮ��Cװ����ʢ��������ϡ���ᣬ������װ��������̼��ط�ĩ��


