��Ŀ����
��A��B��C��D��E����Ԫ�أ���֪AԪ�ص�ԭ������Ȼ����������С��ԭ�ӣ�BԪ�صij�������ռ���������21%��CԪ�ص�һ�ֵ�������Ȼ������Ӳ�����ľ��壻DԪ���Ի���̬�㷺������ʯ��ʯ�ȿ����У����������Ӵ���������λ������ɣ�E�ǵؿ��к������Ľ���Ԫ�أ���ش�
AԪ�صĵ��ʵĻ�ѧʽ��
DԪ�ص������ӵķ�����
BԪ�����γɵij������ʵ�һ����;��
E��B�γɵĻ�����Ļ�ѧʽ��
AԪ�صĵ��ʵĻ�ѧʽ��
H2
H2
��DԪ�ص������ӵķ�����
Ca2+
Ca2+
��BԪ�����γɵij������ʵ�һ����;��
���Ȳ��ˣ���֧��ȼ�գ�
���Ȳ��ˣ���֧��ȼ�գ�
��E��B�γɵĻ�����Ļ�ѧʽ��
Al2O3
Al2O3
������������AԪ�ص�ԭ������Ȼ����������С��ԭ�ӣ�����A������Ԫ�أ�BԪ�صij�������ռ���������21%������B������Ԫ�أ�CԪ�ص�һ�ֵ�������Ȼ������Ӳ�����ľ��壬����C����̼Ԫ�أ�DԪ���Ի���̬�㷺������ʯ��ʯ�ȿ����У����������Ӵ���������λ������ɣ�����D���Ǹ�Ԫ�أ�EԪ���ǵؿ��к������Ľ���Ԫ�أ��ؿ���Ԫ�غ����ɶൽ�ٵ�˳��Ϊ��O��Si��Al��Fe�����к������Ľ���ΪAl������EԪ��ΪAlԪ�أ�
����⣺AԪ�ص�ԭ������Ȼ����������С��ԭ�ӣ�����A������Ԫ�أ�BԪ�صij�������ռ���������21%������B������Ԫ�أ�CԪ�ص�һ�ֵ�������Ȼ������Ӳ�����ľ��壬����C����̼Ԫ�أ�DԪ���Ի���̬�㷺������ʯ��ʯ�ȿ����У����������Ӵ���������λ������ɣ�����D���Ǹ�Ԫ�أ�EԪ���ǵؿ��к������Ľ���Ԫ�أ��ؿ���Ԫ�غ����ɶൽ�ٵ�˳��Ϊ��O��Si��Al��Fe�����к������Ľ���ΪAl������EԪ��ΪAlԪ�أ���ˣ�
AԪ�صĵ��ʵĻ�ѧʽ��H2��DԪ�ص������Ӵ���������λ������ɣ����ķ�����Ca2+��BԪ�����γɵij������������������Լ��Ȳ��ˣ���֧��ȼ�գ���E��B�ֱ�����Ԫ�غ���Ԫ�أ��γɵĻ�����Ļ�ѧʽ��Al2O3��
�ʴ�Ϊ��H2��Ca2+�����Ȳ��ˣ���֧��ȼ�գ���Al2O3��
AԪ�صĵ��ʵĻ�ѧʽ��H2��DԪ�ص������Ӵ���������λ������ɣ����ķ�����Ca2+��BԪ�����γɵij������������������Լ��Ȳ��ˣ���֧��ȼ�գ���E��B�ֱ�����Ԫ�غ���Ԫ�أ��γɵĻ�����Ļ�ѧʽ��Al2O3��
�ʴ�Ϊ��H2��Ca2+�����Ȳ��ˣ���֧��ȼ�գ���Al2O3��
������������Ҫ�����˻�ѧ��һЩ����Ԫ�ص����ã��ڽ������ʱ���ȸ������е���֪�����Ƴ�Ԫ�ص����࣬Ȼ���ٽ�Ԫ��������ʼ��ɽ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

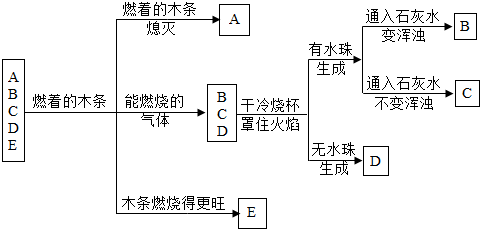

 ��A��B��C��D��E���ֳ������ʣ���������ͼ��ʾ�Ĺ�ϵ����֪��C��һ�ֽ���������D����ʹ����ʯ��ˮ����ǣ�E�����ж������������ڵ�Ѫ�쵰��ϣ��ƶ�B���ʵĻ�ѧʽ��
��A��B��C��D��E���ֳ������ʣ���������ͼ��ʾ�Ĺ�ϵ����֪��C��һ�ֽ���������D����ʹ����ʯ��ˮ����ǣ�E�����ж������������ڵ�Ѫ�쵰��ϣ��ƶ�B���ʵĻ�ѧʽ��