��Ŀ����
 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ�ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ�ش��������⣺
��1����Լˮ��Դ����ֹˮ��Ⱦ��ÿ������Ӧ�������κ������������������ˮ����Ⱦ���У�����ţ�______��
A����ҵ��ˮֱ���ŷš�����B����ҵ�����������ŷ�
C����ֹʹ�ú���ϴ�·ۡ���D������ʹ�û��ʡ�ũҩ
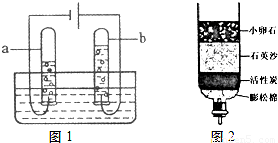
��2����ͼ��ˮͨ��ֽ��ʾ��ͼ����ʵ������У��Թ�a�в�����������______��д���÷�Ӧ�Ļ�ѧ����ʽ��______��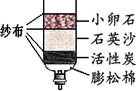
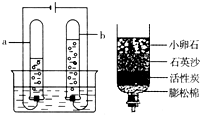
��3��Ϊ��ȥˮ�еIJ��������ʣ�ijͬѧ��������ͼ��ʾ�ļ���ˮ�������л���̿����Ҫ������______��
��4��ijЩ�ط�������ˮ�к���������Ca��HCO3��2�ȿ������Σ���ˮʱ��Ca��HCO3��2�����ֽⷴӦ�����������Ե�CaCO3����д��Ca��HCO3��2���ȷֽ�Ļ�ѧ����ʽ______��
�⣺��1�������ˮ����Ⱦ�������ܶ࣬A����ҵ��ˮֱ���ŷ�D������ʹ�û��ʡ�ũҩ�������ˮ��Ⱦ��
��2�����ˮ��Ӧ�Ļ�ѧ����ʽΪ��2H2O 2H2��+O2�����Թ�a���Դ�ĸ�����������������������
2H2��+O2�����Թ�a���Դ�ĸ�����������������������
��3������̿������ˮ�еIJ��������ʣ�
��4��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O
�ʴ�Ϊ����1��A��D
��2����������H2����2H2O 2H2��+O2��
2H2��+O2��
��3������
��4��Ca��HCO3��2 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O
������������ȷ����ҵ��ˮֱ���ŷţ�����ʹ�û��ʡ�ũҩ�������ˮ�ۣ������ˮҪ������������ŷţ����ˮ��ȡ�����Ļ�ѧ����ʽΪ��2H2O 2H2��+O2�����Թ�a���Դ�ĸ����������������������������û���̿����ˮ�еIJ���������������ˮ��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2
2H2��+O2�����Թ�a���Դ�ĸ����������������������������û���̿����ˮ�еIJ���������������ˮ��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O
���������⿼��ˮ����Ⱦ�뾻�����й�֪ʶ������Ҫ��ȡ���ִ�ʩ����ʵ����ˮ��Ⱦ������ˮԴ��
��2�����ˮ��Ӧ�Ļ�ѧ����ʽΪ��2H2O
 2H2��+O2�����Թ�a���Դ�ĸ�����������������������
2H2��+O2�����Թ�a���Դ�ĸ�������������������������3������̿������ˮ�еIJ��������ʣ�
��4��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2
 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O�ʴ�Ϊ����1��A��D
��2����������H2����2H2O
 2H2��+O2��
2H2��+O2����3������
��4��Ca��HCO3��2
 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O������������ȷ����ҵ��ˮֱ���ŷţ�����ʹ�û��ʡ�ũҩ�������ˮ�ۣ������ˮҪ������������ŷţ����ˮ��ȡ�����Ļ�ѧ����ʽΪ��2H2O
 2H2��+O2�����Թ�a���Դ�ĸ����������������������������û���̿����ˮ�еIJ���������������ˮ��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2
2H2��+O2�����Թ�a���Դ�ĸ����������������������������û���̿����ˮ�еIJ���������������ˮ��Ca��HCO3��2���ȷֽ����ˮ���Ļ�ѧ����ʽΪ��Ca��HCO3��2 CaCO3��+CO2��+H2O
CaCO3��+CO2��+H2O���������⿼��ˮ����Ⱦ�뾻�����й�֪ʶ������Ҫ��ȡ���ִ�ʩ����ʵ����ˮ��Ⱦ������ˮԴ��

��ϰ��ϵ�д�
�����Ŀ
 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺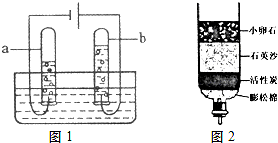 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺