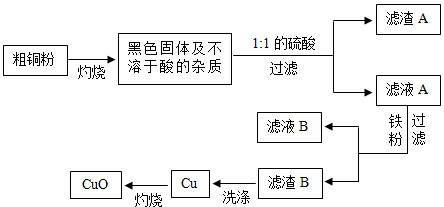
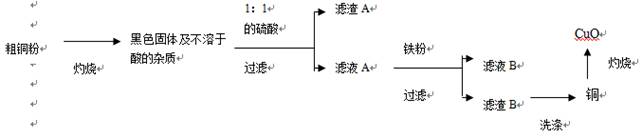
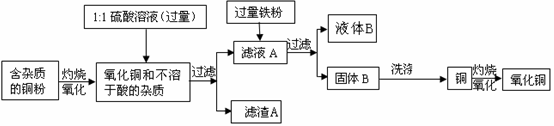
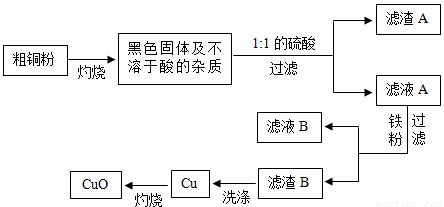
��Ŀ����
�ߴ��ȵ�CuO���������ϡ��л��ϳɴ����ȡ��������ô�ͭ�������������ߴ���CuO������ͼ��˵���������Լ�����������

�ش��������⣺
��1��1:1��H2SO4����1���98%��H2SO4��1���ˮ��϶��ɡ����Ƹ�������Һ����IJ����������������⣬����Ҫ___________________________��
��2��д���������ܽ��ɫ����Ļ�ѧ����ʽ��__________________________��
��3��Ϊ�˴ﵽ��Դ�ۺ����õ�Ŀ�ģ���ϴ������B�õ���ϴ��Һ����ҺB�ϲ�������Ũ����__________��___________�Ȳ��������̷�����(FeSO4•7H2O)��
��4����ϴ�Ӳ����У���______________��д�Լ��Ļ�ѧʽ��ϴ������B��
��5���������������������е���ģ�Ҫ�����ͭ����Cu�ĺ�������Ҫ�ⶨ�������ǣ�������������ͭ��������________________________�������ֱ�ʾ��
��1���ձ�
�� ��Ͳ
����2��CuO+H2SO4═CuSO4+H2O
��3���ᾧ
�� ����
��4��H2SO4
��5�� ��ͭ������

��ϰ��ϵ�д�
�����Ŀ