��Ŀ����
�����Ƹ�����ͭ��Һ�������·�Ӧ��2Na+2H2O+CuSO4�TNa2SO4+Cu��OH��2��+H2������73.7g����ͭ��Һ�м���2.3g�ƣ�ǡ�÷�Ӧ��ȫ���Լ��㣺
��1�����ɳ�����������������Ϊ���ٿˣ�
��2����Ӧֹͣ����ȥ������������Һ�����ʵ����������Ƕ��٣�
��3����˼��ѧϰ��һ����Ҫ���������ݽ����Ƹ�����ͭ��Һ������Ӧ��ʵ�ʣ���Խ������˳������⽫�������̣���̸һ̸��Խ������˳����õ���ʶ��________��
�⣺������������ͭ������Ϊx����������������Ϊy�����������Ƶ�����Ϊz��
2Na+2H2O+CuSO4�TNa2SO4+Cu��OH��2��+H2��
46 142 98 2
2.3g z x y

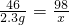
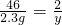
z=7.1g x=4.9g y=0.1g
��1�������ɳ���������Ϊ4.9g����������������Ϊ0.1g��
��2��������Һ�����ʵ���������Ϊ��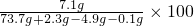 %=10%
%=10%
��������Һ�����ʵ�����������10%��
��3���ڽ������˳���У�����ǰ��Ľ�����һ���ܰѺ���Ľ�����������Һ���û�������
������73.7g������ͭ��Һ������������ͭ��Һ�ǻ������ܴ��뻯ѧ����ʽֱ�Ӽ��㣬2.3g�Dzμӷ�Ӧ���Ƶ��������ɴ��뻯ѧ����ʽ���м��㣮
��1���ɸ����Ƶ������������������ͭ��������������������
��2��������Һ�����ʵ���������=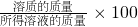 %�������������ƣ��ɸ����Ƶ����������������Һ������=�ձ������ӵ��������ʵ�����-���������-������������
%�������������ƣ��ɸ����Ƶ����������������Һ������=�ձ������ӵ��������ʵ�����-���������-������������
��3����������ѧ�����εĻ�ѧ���ʣ��ƺ�����ͭ��ӦӦ������ͭ�������ƣ���ʵ���ϣ��Ʋ�û�а�ͭ������ͭ��Һ���û�������
�������йط�Ӧ��������Һ�������ļ�����һ���ѵ㣬Ҫ��ѧ�����շ���������������������ʵ�����-����������-���������=������Һ��������
2Na+2H2O+CuSO4�TNa2SO4+Cu��OH��2��+H2��
46 142 98 2
2.3g z x y

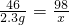
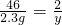
z=7.1g x=4.9g y=0.1g
��1�������ɳ���������Ϊ4.9g����������������Ϊ0.1g��
��2��������Һ�����ʵ���������Ϊ��
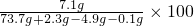 %=10%
%=10%��������Һ�����ʵ�����������10%��
��3���ڽ������˳���У�����ǰ��Ľ�����һ���ܰѺ���Ľ�����������Һ���û�������
������73.7g������ͭ��Һ������������ͭ��Һ�ǻ������ܴ��뻯ѧ����ʽֱ�Ӽ��㣬2.3g�Dzμӷ�Ӧ���Ƶ��������ɴ��뻯ѧ����ʽ���м��㣮
��1���ɸ����Ƶ������������������ͭ��������������������
��2��������Һ�����ʵ���������=
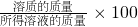 %�������������ƣ��ɸ����Ƶ����������������Һ������=�ձ������ӵ��������ʵ�����-���������-������������
%�������������ƣ��ɸ����Ƶ����������������Һ������=�ձ������ӵ��������ʵ�����-���������-��������������3����������ѧ�����εĻ�ѧ���ʣ��ƺ�����ͭ��ӦӦ������ͭ�������ƣ���ʵ���ϣ��Ʋ�û�а�ͭ������ͭ��Һ���û�������
�������йط�Ӧ��������Һ�������ļ�����һ���ѵ㣬Ҫ��ѧ�����շ���������������������ʵ�����-����������-���������=������Һ��������

��ϰ��ϵ�д�
�����Ŀ
ij��ѧ��ȤС���ͬѧΪ��̽��Mg��Cu��Fe�Ľ������˳��չ��һ������̽������������飬��ý��ۣ��������ʵ�鱨�棬���ش��й����⣮
| ʵ�鷽�� | ʵ������ | ʵ����� | |
| �� �� һ | ��Fe˿����CuSO4��Һ�� | Fe˿�����к�ɫ���������� | ������ԣ�Fe________Cu����������������÷�Ӧ�Ļ�ѧ����ʽΪ��_________�� |
| �� �� �� | ��Cu��Mg�ֱ��ڿ��������� | ����________����Ԫ�ط��ţ��ܾ���ȼ�գ�����һ�ֲ��� | ������ԣ�Cu________Mg������������� |
�� �� �� | �ֱ�Mg��Fe����________��һ���ᣩ��Һ�У��۲����� | �۲쵽________����Ԫ�ط��ţ��������ݵ��ٶȽ��� | �������˳��Mg________Fe��������������� |