��Ŀ����
ˮ������֮Դ��Ҳ�dz�����������Һ���ܼ���
�Ÿ��ݳ�����ѧ֪ʶ����֤��ˮ����H��O��Ԫ����ɵĻ�ѧʵ���� ʵ�飬��ʵ�鷴Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��ij��ѧ�о���ѧϰС��Ը��еĺ�ˮ��ˮ��״��������صĵ����о���
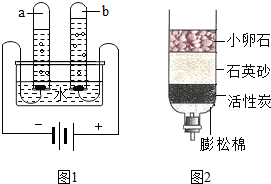
��ȡˮ�������ú���ˡ����˲��������õ�������������©���� �Ͳ�������
����Ҫ�����ˮ����Ӳˮ������ˮ�����õ������� ��
����Ҫ�ⶨ��ˮ�����ȣ���ѡ�� ��������ĸ��
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
�ܹ�����Աÿ�����С���ںӱߴ�������������������Ʒ��������ӦͶ������_____������ĸ������Ͱ�ڡ�

A B  C
C
������Ϊ������Ϊ������ɺ�ˮ���½����� ��������ĸ��
A���������ӱ���ֲ������ľ B������������ˮ����������
C��ijЩ�����������ֶ������
��Ϊ������50g 5%���Ȼ�����Һ����������²������� ���㣻�� ��������ƽ����
_________g �Ȼ��ƣ��� ��__________���10mL����50mL������Ͳ��ȡ______mLˮ���� �����������ձ��У��ò��������衣


 ��2013?Ϋ����ģ��ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
��2013?Ϋ����ģ��ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺ ��
��X�Ļ�ѧʽΪ
���˷�Ӧ��������������� ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص�������
���ѧʽ����
��
��X�Ļ�ѧʽΪ
���˷�Ӧ��������������� ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص�������
���ѧʽ����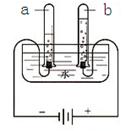

 �ݡ�84������Һ����Ч�ɷ�NaClO�е���Ԫ�صĻ��ϼ�Ϊ (11) ��Cl2��������ˮ����������ʵ�����Ʒ�Ϊ �� ��X�Ļ�ѧʽΪ (12) ���˷�Ӧ��������������� (13) ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص������� ��14�� ���ѧʽ����
�ݡ�84������Һ����Ч�ɷ�NaClO�е���Ԫ�صĻ��ϼ�Ϊ (11) ��Cl2��������ˮ����������ʵ�����Ʒ�Ϊ �� ��X�Ļ�ѧʽΪ (12) ���˷�Ӧ��������������� (13) ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص������� ��14�� ���ѧʽ����