��Ŀ����
�û�ѧ����ʽ��ʾ��������ʵ��صĻ�ѧԭ����
��1����ϡ���������
��2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ�
��3��������ú���̴���ð���������к���SO2������Ⱦ������Ϊʲô����������������Һ�����ո����壿
��4����ʯ���飨�������Ƶ�����Һ��������ͭ��Һ��ϣ��������Ʒ������ѵ����ﲡ���IJ�����Һ������д�������ƹ����з����Ļ�ѧ��Ӧ����ʽ��
��1����ϡ���������
Fe2O3+3H2SO4�TFe2��SO4��3+3H2O
Fe2O3+3H2SO4�TFe2��SO4��3+3H2O
����2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ�
CaCO3+2HNO3�TCa��NO3��2+H2O+CO2��
CaCO3+2HNO3�TCa��NO3��2+H2O+CO2��
����3��������ú���̴���ð���������к���SO2������Ⱦ������Ϊʲô����������������Һ�����ո����壿
2NaOH+SO2�TNa2SO3+H2O
2NaOH+SO2�TNa2SO3+H2O
����4����ʯ���飨�������Ƶ�����Һ��������ͭ��Һ��ϣ��������Ʒ������ѵ����ﲡ���IJ�����Һ������д�������ƹ����з����Ļ�ѧ��Ӧ����ʽ��
CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4
CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4
����������1����ϡ������������������������Ӧ������������ˮ��
��2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ��������̼��Ʒ�Ӧ��������ơ�ˮ�Ͷ�����̼��
��3���������������������Һ��Ӧ�����������ƺ�ˮ��
��4��ʯ���飨�������Ƶ�����Һ��������ͭ��Һ�������������ͭ����������ƣ�
��2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ��������̼��Ʒ�Ӧ��������ơ�ˮ�Ͷ�����̼��
��3���������������������Һ��Ӧ�����������ƺ�ˮ��
��4��ʯ���飨�������Ƶ�����Һ��������ͭ��Һ�������������ͭ����������ƣ�
����⣺��1����ϡ������������������������Ӧ������������ˮ����ѧ����ʽΪ��Fe2O3+3H2SO4�TFe2��SO4��3+3H2O��
��2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ��������̼��Ʒ�Ӧ��������ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HNO3�TCa��NO3��2+H2O+CO2����
��3���������������������Һ��Ӧ�����������ƺ�ˮ����ѧ����ʽΪ��2NaOH+SO2�TNa2SO3+H2O��
��4��ʯ���飨�������Ƶ�����Һ��������ͭ��Һ�������������ͭ����������ƣ���ѧ����ʽΪ��CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
�ʴ�Ϊ����1��Fe2O3+3H2SO4�TFe2��SO4��3+3H2O����2��CaCO3+2HNO3�TCa��NO3��2+H2O+CO2������3��2NaOH+SO2�TNa2SO3+H2O����4��CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
��2����ϡ�����ˮ����ˮ������Ҫ�ɷ���̼��ƣ��������̼��Ʒ�Ӧ��������ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HNO3�TCa��NO3��2+H2O+CO2����
��3���������������������Һ��Ӧ�����������ƺ�ˮ����ѧ����ʽΪ��2NaOH+SO2�TNa2SO3+H2O��
��4��ʯ���飨�������Ƶ�����Һ��������ͭ��Һ�������������ͭ����������ƣ���ѧ����ʽΪ��CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
�ʴ�Ϊ����1��Fe2O3+3H2SO4�TFe2��SO4��3+3H2O����2��CaCO3+2HNO3�TCa��NO3��2+H2O+CO2������3��2NaOH+SO2�TNa2SO3+H2O����4��CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
�����������ص��ǿ���ѧ����д��ѧ����ʽ�ļ��ɣ�����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

��ϰ��ϵ�д�
�����Ŀ

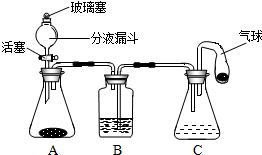 ��2012?��ƽ��һģ����ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������Ȥʵ�飮��Һ©����Cװ������ʢ�ŵ���Һ��ͬ��B��Cװ����ʢ�ŵ���Һ������������װ�������İ�ɫ�����ĩ��
��2012?��ƽ��һģ����ѧС��ͬѧ����ͼ��ʾװ�ã��г���������ȥ������Ȥʵ�飮��Һ©����Cװ������ʢ�ŵ���Һ��ͬ��B��Cװ����ʢ�ŵ���Һ������������װ�������İ�ɫ�����ĩ��