��Ŀ����
��6�֣�ˮ����Һ������������������Ҫ�����á�
��1��ˮ��һ��������ܼ����������������ʷֱ����ˮ�У������γ���Һ���� ����
��ĸ��ţ���
��2�����ˮʵ��֤����ˮ�� ��ɡ�
��3�������п����� ����ijˮ����Ӳˮ������ˮ��
��4����ҵ�����г�����10%��ϡ�����������⣬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
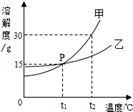
��5��20��ʱ�����������ļס��ҹ��壬�ֱ���뵽ʢ��100gˮ���ձ��У���ֽ����������ͼ1�����ȵ�50��ʱ������ͼ2��������ˮ�����������ס��ҹ����ܽ��������ͼ3��

����˵����ȷ���� ������ĸ��ţ���
ͼ3��N��ʾ���Ǽ��ܽ������
ͼ2�еļ���Һ�Dz�������Һ
ͼ2�мס�����Һ�����������������
��ͼ2������Һ������30��һ����������������
ͼ1�м���Һ��������������������Һ������������
��1��ˮ��һ��������ܼ����������������ʷֱ����ˮ�У������γ���Һ���� ����
��ĸ��ţ���
| A��ֲ���� | B��ʳ�� | C����� | D������ |
��3�������п����� ����ijˮ����Ӳˮ������ˮ��
��4����ҵ�����г�����10%��ϡ�����������⣬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��20��ʱ�����������ļס��ҹ��壬�ֱ���뵽ʢ��100gˮ���ձ��У���ֽ����������ͼ1�����ȵ�50��ʱ������ͼ2��������ˮ�����������ס��ҹ����ܽ��������ͼ3��

����˵����ȷ���� ������ĸ��ţ���
ͼ3��N��ʾ���Ǽ��ܽ������
ͼ2�еļ���Һ�Dz�������Һ
ͼ2�мס�����Һ�����������������
��ͼ2������Һ������30��һ����������������
ͼ1�м���Һ��������������������Һ������������
��1��AC ;��2�� ��Ԫ�غ���Ԫ�� ��3������ˮ ��4�� 6HCl + Fe2O3=== 2FeCl3 + 3H2O��5��BCD
�����������1��ˮ��һ�ֳ������ܼ��������γ���Һ����ֲ���ͺ���۶��߲�����ˮ����2�����ˮʵ��֤����ˮ����Ԫ�غ���Ԫ����ɵģ���3�������п����÷���ˮ����ijˮ����Ӳˮ������ˮ����4����ҵ�����г�����10%��ϡ�����������⣬�÷�Ӧ�Ļ�ѧ����ʽΪ6HCl + Fe2O3=== 2FeCl3 + 3H2O����5��������ͼʾ���ܽ�����߿�֪��˵����ȷ����BCD

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ