��Ŀ����
��8�֣���������ʵ�鳣��װ�ã��ش��й����⡣
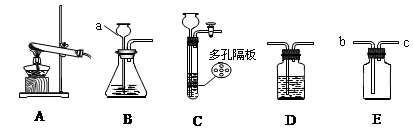
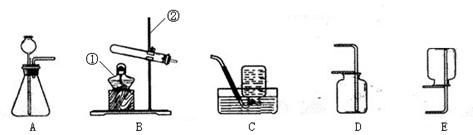
(1)д��ͼ�б�����ĸ�����������ƣ�a__________��
(2)ʵ�����ø��������ȡ������Ӧѡ�õķ���װ����_________���ڼ���ҩƷǰ��Ӧ���ȼ��װ�õ� ��
(3)��Ҫ��ȡ������̼���仯ѧ����ʽΪ ����Ҫ��ø���Ķ�����̼��������װ���⣬��Ӧѡ��Dװ�ã����ڸ�װ����ʢ�� ����д�Լ����ƣ��������Eװ���ռ������壬�������_________�˽��루�b����c������
(4)ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ��� ��

(1)д��ͼ�б�����ĸ�����������ƣ�a__________��
(2)ʵ�����ø��������ȡ������Ӧѡ�õķ���װ����_________���ڼ���ҩƷǰ��Ӧ���ȼ��װ�õ� ��
(3)��Ҫ��ȡ������̼���仯ѧ����ʽΪ ����Ҫ��ø���Ķ�����̼��������װ���⣬��Ӧѡ��Dװ�ã����ڸ�װ����ʢ�� ����д�Լ����ƣ��������Eװ���ռ������壬�������_________�˽��루�b����c������
(4)ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ��� ��
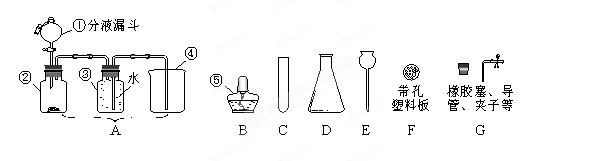
(1)����©����2��A ������ ��3�� CaCO3 + 2HCl=CaCl2+H2O +CO2��
Ũ���� b ��4������ʹ��Ӧ��ʱ���У�Ҳ����ʹ��Ӧ��ʱֹͣ
Ũ���� b ��4������ʹ��Ӧ��ʱ���У�Ҳ����ʹ��Ӧ��ʱֹͣ
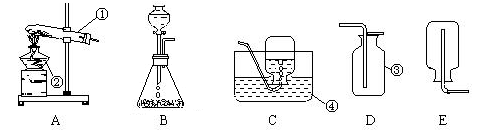
��1��ֱ��д������������Ϊ����©����
��2����������ǹ��壬���ø��������ȡ����ʱ��Ҫ���ȣ�����Ӧ��ѡ��Zװ������Ϊ����װ�ã��ڼ���������֮ǰҪ��֤װ�õ������������õģ����Ա������ȼ��װ�õ�������;
��3������©�������þ��Ƿ�������ƿ�м���ϡ����ģ�������̼�����������壬����Ӧ��ѡ��Ũ�������������Dװ����Ӧ�ü���Ũ���ᣬ���ڶ�����̼���ܶȱȿ����Ĵ���������Eװ�����ռ�������̼ʱӦ�ô�b�ܽ��룬ʵ��������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��4����װ��C�м����˶���壬����ͨ�����ɼ������Ʒ�Ӧ������Ҫ��ʱ����ʱ��ʼ����ֹʵ�飮
��2����������ǹ��壬���ø��������ȡ����ʱ��Ҫ���ȣ�����Ӧ��ѡ��Zװ������Ϊ����װ�ã��ڼ���������֮ǰҪ��֤װ�õ������������õģ����Ա������ȼ��װ�õ�������;
��3������©�������þ��Ƿ�������ƿ�м���ϡ����ģ�������̼�����������壬����Ӧ��ѡ��Ũ�������������Dװ����Ӧ�ü���Ũ���ᣬ���ڶ�����̼���ܶȱȿ����Ĵ���������Eװ�����ռ�������̼ʱӦ�ô�b�ܽ��룬ʵ��������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��4����װ��C�м����˶���壬����ͨ�����ɼ������Ʒ�Ӧ������Ҫ��ʱ����ʱ��ʼ����ֹʵ�飮

��ϰ��ϵ�д�
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
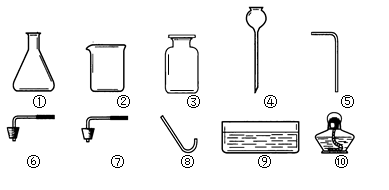

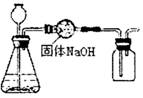
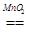 2H2O��O2��
2H2O��O2��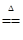 CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O