��Ŀ����
(10��)ij�ظ��ݵ�����Դ�ص㽫ʯ��ʯ���ɡ��ӹ���¯��������һ���γ�������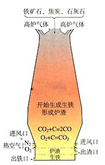
(1)������ԭ��������ʯ����̿��ʯ��ʯ�Ϳ�������̿����Ҫ���������㣺��һ�����ɻ�ԭ��CO������� ��д���������ڼ���ʱ����ԭ�����ķ���ʽ
(2)���������У�����ԭ�ϴ�¯�����¼ӣ����ȿ���ȴ�Ǵ�¯��ͨ�롣���������ŵ��� ��
��¯�����ŷŵķ����к��д����ĸ���һ����̼������Ϊ��Դ��������ʯ��ʯ����д������ʯ��ʯ�Ļ�ѧ����ʽ�� ��
(3)��������ʽ����Ҫ�ŵ��� (���һ�㼴��)
��1��ȼ�շ��ȣ�ά��¯�¡���Fe2O3 + 3CO 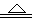 2Fe + 3CO2
2Fe + 3CO2
��2��ʹ��Ӧ���ֽӴ� CaCO3 CaO + CO2��
CaO + CO2��
��3�����ͳɱ�(����Ϊ�������ٻ�����Ⱦ����Լ��Դ������������Դ�ȣ����������𰸾�����)
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
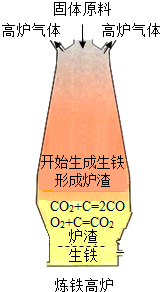
 ij�ظ��ݵ�����Դ�ص㽫ʯ��ʯ���ɡ��ӹ���¯��������һ���γ�������
ij�ظ��ݵ�����Դ�ص㽫ʯ��ʯ���ɡ��ӹ���¯��������һ���γ�������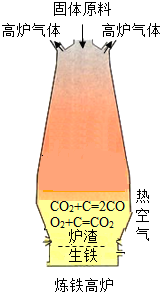 ij�ظ��ݵ�����Դ�ص㽫ʯ��ʯ���ɡ��ӹ���¯��������һ���γ�������
ij�ظ��ݵ�����Դ�ص㽫ʯ��ʯ���ɡ��ӹ���¯��������һ���γ�������