��Ŀ����
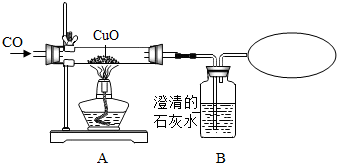
ij����С�飬����һ����̼����ⶨCuO�Ļ�ԭ�ʣ�ʵ�鿪ʼʱ���Բ���ʵ�������δ���κδ�����ֱ��ʹ�ã������õ�CuO�Ļ�ԭ�ʳ���100%�����������������۲��Ľ�ʵ��װ�ã��Ľ����ʵ��װ�ü���õ�ʵ�����ݼ���ͼ����

�Իش�
��1���Ľ�ʵ��װ��ǰ����õ�CuO�Ļ�ԭ�ʳ�����100%������ܵ�ԭ��֮һ��ͨ���CO�����л���______���壮
��2�������ɵ�������Dװ���б���ȫ���գ����õ�CuO�Ļ�ԭ��Ϊ______��
��3���˸Ľ���ʵ��װ�����в����Ƶĵط���������Ľ�����______��

| ������������g�� | 7 |
| CuO������g�� | 20 |
| ����������g�� | 20 |
��1���Ľ�ʵ��װ��ǰ����õ�CuO�Ļ�ԭ�ʳ�����100%������ܵ�ԭ��֮һ��ͨ���CO�����л���______���壮
��2�������ɵ�������Dװ���б���ȫ���գ����õ�CuO�Ļ�ԭ��Ϊ______��
��3���˸Ľ���ʵ��װ�����в����Ƶĵط���������Ľ�����______��
��1��һ����̼��ԭ����ͭ���ɶ�����̼��Dװ���е�ʯ��ˮ���գ����Լ����CuO�Ļ�ԭ�ʣ��������������ж�����̼��Ҳ�ᱻD�е�ʯ��ˮ���գ���������CuO�Ļ�ԭ�ʾͻᳬ��100%��
�ʴ�Ϊ��������̼��
��2�������ɵĶ�����̼������Ϊx��
CO2+Ca��OH��2�TCaCO3��+H2O
44 100
x 20g
=
x=8.8g
�豻��ԭ������ͭ������Ϊy��
CO+CuO
Cu+CO2
80 44
y 8.8g
=
y=16g��
CuO�Ļ�ԭ��Ϊ��
��100%=80%��
�ʴ�Ϊ��80%��
��3�����������һ����̼�ж��������ų�װ���⣬����Ҫ����β��������һ����̼����ȼ�գ����Կ��Խ�β����ȼ���ռ����������õȣ�
�ʴ�Ϊ��Ӧ��β�����д��������Խ�β����ȼ���ռ����������õȣ�
�ʴ�Ϊ��������̼��
��2�������ɵĶ�����̼������Ϊx��
CO2+Ca��OH��2�TCaCO3��+H2O
44 100
x 20g
| 44 |
| 100 |
| x |
| 20g |
x=8.8g
�豻��ԭ������ͭ������Ϊy��
CO+CuO
| ||
80 44
y 8.8g
| 80 |
| 44 |
| y |
| 8.8g |
y=16g��
CuO�Ļ�ԭ��Ϊ��
| 16g |
| 20g |
�ʴ�Ϊ��80%��
��3�����������һ����̼�ж��������ų�װ���⣬����Ҫ����β��������һ����̼����ȼ�գ����Կ��Խ�β����ȼ���ռ����������õȣ�
�ʴ�Ϊ��Ӧ��β�����д��������Խ�β����ȼ���ռ����������õȣ�

��ϰ��ϵ�д�
�����Ŀ