��Ŀ����
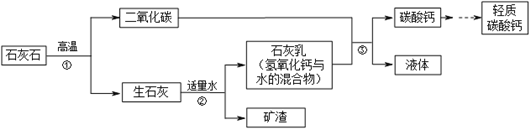
����Ŀ������̼��Ƶijɷ���CaCO3������ζ�İ�ɫ��ĩ����Ӧ����ͭ��ֽ����Ʊֽ��ֽ��Ʒ�У��������ֽ��Ʒ���ȶ��ԡ�Ӳ�Ⱥ������ԡ����ڹ��ڵĹ�ҵ����������Ҫ��̼�����������������£�
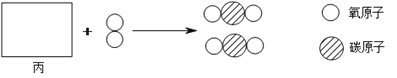
��1�����������IJ���������_______________��
��2�����������У��������Ϸ�Ӧ�Ļ�ѧ����ʽΪ________________��
��3��ʯ��ʯ������̼��ƵIJ���ǣ�д��һ����_________________��
��4����ʯ����һ�ָ���������Ը����������_______________��
A��N2 B��O2 C��CO2 D��HCl
��5������ij�����Ҳ�ᶨ������������ʯ�ң����Լ������༲���ķ�����������ʯ�Һ����ˮ��pH_________���������������������7��
���𰸡� ���� CaO+ H2O��Ca(OH)2 ʯ��ʯ�ǻ�������̼��Ƹ��� AB >
��������������Ҫ�����̼����йص�֪ʶ��ʯ��ʯ����Ҫ�ɷ�Ϊ̼��ƣ��ڸ��µ������·ֽ�������ƺͶ�����̼�������ƺ�ˮ��Ӧ�����������ƣ����������������̼��Ӧ������̼��ƺ�ˮ��
��1������ۺ�õ������Һ�壬���Բ������ƣ�����
��2�����ݷ����������ƺ�ˮ��Ӧ�������������ǻ��Ϸ�Ӧ����ѧ����ʽΪ��
CaO+ H2O==Ca(OH)2
��3��ʯ��ʯ�е����ʣ�����̼����Ǵ��������ʯ��ʯ������̼��ƵIJ��������̼��Ƹ���
��4����ʯ������ˮ��Ӧ�����������ƣ������������ڼ������ʯ�ҿ��Ը����������A��B
��5����ʯ������ˮ��Ӧ�����������ƣ������������ڼ����pH ��7.