��Ŀ����
�Ķ����ж��ģ������ṩ����Ϣ����ѧ����֪ʶ�ش����⡣�ݱ������������ѧ���ڰ�Ľ�����ǵ����������һ������Դ���������ʵ���۲�����ֳ�����ȼ������ѧ���о���������ȼ��ʵ������һ�ּ���ˮ���CH4•XH2O�����Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�һ�ֹ�̬���ʡ�
(1)��ȼ����һ�־��壬������ƽ��ÿ46��H2O����8������ÿ�����ڿ�����1��CH4��һ�������H2O����������ÿ8������6��������CH4������2�����������H2O��䡣���ȼ����ƽ����ɿɱ�ʾΪ ( )
A��2CH4•H2O������������ ������������ B��CH4•3H20
C��CH4•6H2O���������������������������� D��CH4•8H2O
(2)Ŀǰ���ŷŵ������е�������Ⱦ����Ҫ��SO2��NO2��CO�ȣ�������Ҫ���Կ���ȼ�ϵ�ȼ�պ�ҵ����������a����ȼ����b�����ܡ�c�����ܡ�d��̫���ܡ�e��ú��f����Ȼ����g��ʯ�͵���Դ����ͨ�������ʹ��������Դ��һ�㲻����ɿ�����Ⱦ����________��
(3)��ȼ���ķ���Ϊ�ҹ���������ʹ�ø�Ч��Դ�����˹�����ǰ��������Ϊ�ܿ������õ���Դ���У���������֣�_____���������������� ___��
������
| (1)D
(2)a��b��c��d��f (3)���ȡ���ϫ�ܡ����ܵȸ�Ч����Ⱦ��Դ
|
��ʾ��
| (1)��ȼ���ľ�����ƽ��ÿ46��H2O����8������ÿ�����ڿ�����1��CH4��һ�������H2O����������ÿ8������6��������CH4����2�����������H2O���ʱ���������ӣ�ˮ���Ӹ���֮��=6��48=1��8��
(2)����ɴ�����Ⱦ��ú��ʯ�ͣ������߾�Ϊ��̼���ܸߵĻ����ײ���ȫȼ�ղ���һ����̼��̿����ú�к���Ԫ��ȼ�ջ������������ (3)���ȡ���ϫ�ܡ����ܵȸ�Ч����Ⱦ��Դ
|

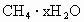 )���Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�һ�ֹ�̬���ʣ�
)���Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�һ�ֹ�̬���ʣ�