��Ŀ����
�ס��ҡ�����������ֱ���ϡ���ᡢþ������������Һ������þ������ͭ��Һ�е�һ�֡���ͼ��ӳ������֮������ϵ�����С�һ����ʾ���������ʼ�������Ӧ����������ʾ ����һ�������¿���ת��Ϊ�졣��֪����Һ����ɫ����ش��������⣺

(1)д����������(������)�Ļ�ѧʽ����_____________����__________��
(2)ͼ�и���Ӧ���������ֻ�����Ӧ�����е�________��Ӧ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ���������______________�����붡��_________________��
(4)���ٱ��������������е�һ����;��______________��
(2)ͼ�и���Ӧ���������ֻ�����Ӧ�����е�________��Ӧ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ���������______________�����붡��_________________��
(4)���ٱ��������������е�һ����;��______________��
(1)Mg��MgO
(2)�ֽ�
(3)2NaOH+H2SO4==Na2SO4+2H2O��2NaOH+CuSO4==Na2SO4+Cu(OH)2��
(4)��ijЩ����ĸ����(��Ӧ���ڷ�������ֽ����֯��ӡȾ�ȹ�ҵ)(�����𰸺���Ҳ��)
(2)�ֽ�
(3)2NaOH+H2SO4==Na2SO4+2H2O��2NaOH+CuSO4==Na2SO4+Cu(OH)2��
(4)��ijЩ����ĸ����(��Ӧ���ڷ�������ֽ����֯��ӡȾ�ȹ�ҵ)(�����𰸺���Ҳ��)

��ϰ��ϵ�д�
�����Ŀ
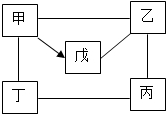 �ס��ҡ�����������ֱ���ϡ���ᡢþ������������Һ������þ������ͭ��Һ�е�һ�֣�
�ס��ҡ�����������ֱ���ϡ���ᡢþ������������Һ������þ������ͭ��Һ�е�һ�֣� 10������ͼ��ʾ�����мס��ҡ�����������ֱ�������ͭ��ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ����������Բ��ʾ��Ӧ�����ܷ�����Ӧ����֪�Ͷ���Ӧ�����ݳ��֣�������˵����ȷ���ǣ�������
10������ͼ��ʾ�����мס��ҡ�����������ֱ�������ͭ��ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ����������Բ��ʾ��Ӧ�����ܷ�����Ӧ����֪�Ͷ���Ӧ�����ݳ��֣�������˵����ȷ���ǣ������� ��2013?��ɽ����ģ����ͼ�����мס��ҡ�����������ֱ�����������ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ�������������ܷ�����Ӧ����֪�Ͷ���Ӧ�����ݳ��֣�������˵����ȷ���ǣ�������
��2013?��ɽ����ģ����ͼ�����мס��ҡ�����������ֱ�����������ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ�������������ܷ�����Ӧ����֪�Ͷ���Ӧ�����ݳ��֣�������˵����ȷ���ǣ������� ��2012?��ˮ�ض�ģ����ͼ��ʾ�����мס��ҡ�����������ֱ�������ͭ��ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ����������Բ��ʾ��Ӧ�����ܷ�����Ӧ��������˵��������ǣ�������
��2012?��ˮ�ض�ģ����ͼ��ʾ�����мס��ҡ�����������ֱ�������ͭ��ϡ���ᡢ����������Һ��̼������Һ��������̼�е�һ�֣�ͼ����������Բ��ʾ��Ӧ�����ܷ�����Ӧ��������˵��������ǣ�������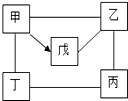 �ס��ҡ�����������ֱ���ϡ���ᡢþ������������Һ������þ������ͭ��Һ�е�һ�֣���ͼ��ӳ������֮������ϵ�����С�-����ʾ���������ʼ�������Ӧ����������ʾ����һ�������¿���ת��Ϊ�죮��֪����Һ����ɫ����ش��������⣺
�ס��ҡ�����������ֱ���ϡ���ᡢþ������������Һ������þ������ͭ��Һ�е�һ�֣���ͼ��ӳ������֮������ϵ�����С�-����ʾ���������ʼ�������Ӧ����������ʾ����һ�������¿���ת��Ϊ�죮��֪����Һ����ɫ����ش��������⣺