��Ŀ����
���ٶ�����̼�ŷţ�����ȫ�������ů�ǵ�������Ҫ������ش����⣮
��1�������������ж�����̼����������������Ҫԭ����
��2�����б仯�У���ά�ִ����ж�����̼��������ƽ��������Ҫ����
A.6CO2+6H2O
C6H12O6+6O2 B��CO2+C
2CO
��3����ѧ�Ҳ����¼�������������̼��������һ��������ϣ���һ�������·��������·�Ӧ��2CO2+6H2=4H2O+X����X�Ļ�ѧʽΪ
��4��Ϊ���������ж�����̼���������ӣ����½�����е���
�ٿ���̫���ܡ�ˮ�ܡ����ܵ�����Դ���ڽ�ֹʹ��ú��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ��۴���ֲ�����֣���ֹ�ҿ��ķ���
��5����Щ���еij���Ѹ���ѹ����Ȼ����ȼ�ϣ��Լ��ٶԿ�����Ⱦ����Ȼ����ȫȼ�յĻ�ѧ����ʽΪ
��1�������������ж�����̼����������������Ҫԭ����
��ʯȼ�ϵĴ���ȼ��
��ʯȼ�ϵĴ���ȼ��
����2�����б仯�У���ά�ִ����ж�����̼��������ƽ��������Ҫ����
A
A
������ţ�A.6CO2+6H2O
| ||
| ||
��3����ѧ�Ҳ����¼�������������̼��������һ��������ϣ���һ�������·��������·�Ӧ��2CO2+6H2=4H2O+X����X�Ļ�ѧʽΪ
C2H4
C2H4
����4��Ϊ���������ж�����̼���������ӣ����½�����е���
�٢�
�٢�
������ţ����ٿ���̫���ܡ�ˮ�ܡ����ܵ�����Դ���ڽ�ֹʹ��ú��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϣ��۴���ֲ�����֣���ֹ�ҿ��ķ���
��5����Щ���еij���Ѹ���ѹ����Ȼ����ȼ�ϣ��Լ��ٶԿ�����Ⱦ����Ȼ����ȫȼ�յĻ�ѧ����ʽΪ
CH4+2O2
2H2O+CO2
| ||
CH4+2O2
2H2O+CO2
��
| ||
��������1�����ݴ����ж�����̼����Դ������
��2��������ö�ά�ִ����ж�����̼��������ƽ������Ҫ���ã�
��3�����������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ���������
��4�������йصĴ�ʩ�ܷ���������ж�����̼���������ӣ�
��5����������ȼ�յķ�Ӧд����Ӧ�ķ���ʽ��
��2��������ö�ά�ִ����ж�����̼��������ƽ������Ҫ���ã�
��3�����������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ���������
��4�������йصĴ�ʩ�ܷ���������ж�����̼���������ӣ�
��5����������ȼ�յķ�Ӧд����Ӧ�ķ���ʽ��
����⣺��1�������������ж�����̼����������������Ҫԭ���ǻ�ʯȼ�ϵĴ���ȼ�գ�
��2�����ڹ�����ö�ά�ִ����ж�����̼��������ƽ������Ҫ���ã���ѡA��
��3����2CO2+6H2=4H2O+X�У�����ʽ����ߺ���2��̼ԭ�ӡ�4����ԭ�Ӻ�12����ԭ�ӣ��ұ���4����ԭ�Ӻ�8����ԭ�ӣ����1��δ֪����������к���2��̼ԭ�Ӻ�4����ԭ�ӣ���δ֪������Ļ�ѧʽ��ʾΪC2H4��
��4��Ϊ���������ж�����̼���������ӣ����Բ�ȡ����̫���ܡ�ˮ�ܡ����ܵ�����Դ������ֲ�����֣���ֹ�ҿ��ķ��ȴ�ʩ��
��4����Ȼ������Ҫ�ɷ��Ǽ��飮������ȫȼ�յĻ�ѧ����ʽΪ��CH4+2O2
2H2O+CO2��
�ʴ�Ϊ����1����ʯȼ�ϵĴ���ȼ�գ���2��A����3��C2H4����4���٢ۣ���5��CH4+2O2
2H2O+CO2��
��2�����ڹ�����ö�ά�ִ����ж�����̼��������ƽ������Ҫ���ã���ѡA��
��3����2CO2+6H2=4H2O+X�У�����ʽ����ߺ���2��̼ԭ�ӡ�4����ԭ�Ӻ�12����ԭ�ӣ��ұ���4����ԭ�Ӻ�8����ԭ�ӣ����1��δ֪����������к���2��̼ԭ�Ӻ�4����ԭ�ӣ���δ֪������Ļ�ѧʽ��ʾΪC2H4��
��4��Ϊ���������ж�����̼���������ӣ����Բ�ȡ����̫���ܡ�ˮ�ܡ����ܵ�����Դ������ֲ�����֣���ֹ�ҿ��ķ��ȴ�ʩ��
��4����Ȼ������Ҫ�ɷ��Ǽ��飮������ȫȼ�յĻ�ѧ����ʽΪ��CH4+2O2
| ||
�ʴ�Ϊ����1����ʯȼ�ϵĴ���ȼ�գ���2��A����3��C2H4����4���٢ۣ���5��CH4+2O2
| ||
������������Ҫ�����������������̼��ͬѧ���˽��˶�����̼�����������Ҫԭ���Լ���μ��ٶ�����̼���壬�ͻ����ô��⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 2010��12��14�����磬�ԡ���̼���Ϊ�����ר��չ�����й��Ƽ�����ʽ��չ������չ��ּ���������ڸı����������ٶ�����̼�ŷţ�չ������5��������չ����
2010��12��14�����磬�ԡ���̼���Ϊ�����ר��չ�����й��Ƽ�����ʽ��չ������չ��ּ���������ڸı����������ٶ�����̼�ŷţ�չ������5��������չ����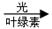 C6H12O6 + 6O2
C6H12O6 + 6O2
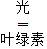 C6H12O6+6O2
C6H12O6+6O2
 C6H12O6+6O2
C6H12O6+6O2