题目内容
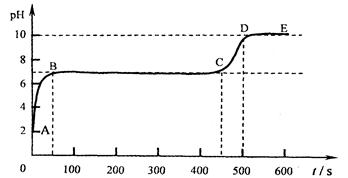
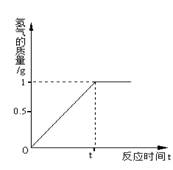
黄铜主要是由铜和锌组成的铜锌合金,它强度高,可塑性好,易加工,耐腐蚀。使用范围广。化学实验小组的同学想知道某种黄铜中铜和锌的含量,他们将100g黄铜磨成粉沫,然后放入盛有足量稀盐酸的烧杯中,反应生成氢气的量与时间的关系如图。
试回答:
(1)反应前将黄铜打磨成粉沫的目的是 。
(2) 该黄铜中铜和锌的质量分数是多少。
(3)若该黄铜中还含有锡(Sn)、铅(Pb)等金属,对计算结果中铜的含量造成的影响是 (填“偏高”或“偏低”)

试回答:
(1)反应前将黄铜打磨成粉沫的目的是 。
(2) 该黄铜中铜和锌的质量分数是多少。
(3)若该黄铜中还含有锡(Sn)、铅(Pb)等金属,对计算结果中铜的含量造成的影响是 (填“偏高”或“偏低”)
(1) 加快反应速率,使反应更彻底 (2)67.5% (3) 偏低
试题分析:(1)反应前将黄铜打磨成粉沫的目的是:增大反应物的接触面积,加快反应速率,使反应更彻底
(2)根据图像,可以得出这样的信息,当反应结束后生成氢气的质量为1g,结合化学反应:Zn + 2HCl="==" ZnCl2 + H2↑中Zn和H2的质量关系65:2,可求出锌的质量,进而可算出该黄铜中铜和锌的质量分数
设:合金中锌的质量为X
Zn+2HCl==ZnCl2+H2↑
65 2
X 1g
65:2==x:1g
X=32.5g
黄铜中锌的质量分数是
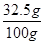 ×100%=32.5%
×100%=32.5%则黄铜中铜的质量分数是1-32.5%=67.5%
(3)因为黄铜中含有的锡(Sn)、铅(Pb)金属,也会与盐酸反应,产生氢气,这样使得氢气质量偏大,算出的锌质量也偏大,故铜的含量造成的影响是偏低

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目