��Ŀ����
 �����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�
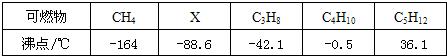
�����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ���1�������dz����г��õĴ��ߣ�����˵��һ����ֹ��������ķ�����
���ֽྻ����
���ֽྻ����
����2��С���������ϸ���ʱ������һЩʳ�ף������ϸ�������ˣ��������Dz����ɺ��أ�������ȡ�ϸ�����֭Һ�ظ���ʳ�ף�������ͬ���������ϸ���֭Һ�м���һЩ�����������ϸ���֭Һ���̣������С��һ�����������⣺
��ʳ��pH
��
��
7���������=����������������������
��
����ᡱ��������Ρ��������ڽ��С����ʵ�飬���������ϸ���֭Һ����;
�����ָʾ��
�����ָʾ��
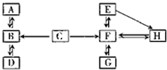
����3��A-H�dz��л�ѧ�еij������ʣ���������֮���ת����ϵͼ���ش��������⣮��ͼ�С�������ʾ���ʼ����ת����ϵ��
��A�ڱ�״�������ܶ���С�����壬A�Ļ�ѧʽ��
H2
H2
��A��B��C�к���ͬһ��Ԫ�أ�C��Bʱ��Һ�ʻ�ɫ���ɴ��ƶ���C��Ӧ����B��������Fe2O3
Fe2O3
����DΪ���嵥�ʣ�д��B��D�Ļ�ѧ����ʽ
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
��
| ||
��������1�����ݷ�ֹ������Ĵ�ʩ���з������
��2����ʳ�׳����ԣ�������̼���ƣ��������ࣻ�ڸ���֭�ڲ�ͬ���������Һ�л���ֲ�ͬ����ɫ����ָʾ�����������ƣ�
��3�������ܶ���С�����壬�����Ƴ�A����������C��Bʱ��Һ�ʻ�ɫ���Ƴ���Һ�к��������ӣ�Ȼ���ץ��A��B��C�к���ͬһ��Ԫ�ء���һ�������Ƴ�CΪ���ᣮ
��2����ʳ�׳����ԣ�������̼���ƣ��������ࣻ�ڸ���֭�ڲ�ͬ���������Һ�л���ֲ�ͬ����ɫ����ָʾ�����������ƣ�
��3�������ܶ���С�����壬�����Ƴ�A����������C��Bʱ��Һ�ʻ�ɫ���Ƴ���Һ�к��������ӣ�Ȼ���ץ��A��B��C�к���ͬһ��Ԫ�ء���һ�������Ƴ�CΪ���ᣮ
����⣺��1������������������ɷ�ֹ�������⣬����������ˮ�ֿɷ�ֹ�������⣬�籣�ֽྻ���
�ʴ�Ϊ�����ֽྻ���
��2���ٴ������Һ�����ԣ�������pHС��7�����ڴ����ǽ�����������������γɣ������������࣮
������Ŀ��Ϣ��֪��������Բ�ͬ����Һ����ɫ����֭Һ����ʾ��ͬ����ɫ�����Կ��������������ָʾ����������ʵ�����Եȣ�
�ʴ�Ϊ���٣����Σ��������ָʾ����
��3�������ܶ���С�����壬�����Ƴ�A����������C��Bʱ��Һ�ʻ�ɫ���Ƴ���Һ�к��������ӣ������C��Ӧ����B�����������������ٸ��ݡ�A��B��C�к���ͬһ��Ԫ�ء����Ƴ�BΪˮ��C�к�����Ԫ�أ������뺬���Ļ����ﷴӦ������CΪ���DΪ���嵥�ʣ�BΪˮ�����Ƴ�DΪ������
�ʴ�Ϊ��
��H2��Fe2O3����2H2O
2H2��+O2����
�ʴ�Ϊ�����ֽྻ���
��2���ٴ������Һ�����ԣ�������pHС��7�����ڴ����ǽ�����������������γɣ������������࣮
������Ŀ��Ϣ��֪��������Բ�ͬ����Һ����ɫ����֭Һ����ʾ��ͬ����ɫ�����Կ��������������ָʾ����������ʵ�����Եȣ�
�ʴ�Ϊ���٣����Σ��������ָʾ����
��3�������ܶ���С�����壬�����Ƴ�A����������C��Bʱ��Һ�ʻ�ɫ���Ƴ���Һ�к��������ӣ������C��Ӧ����B�����������������ٸ��ݡ�A��B��C�к���ͬһ��Ԫ�ء����Ƴ�BΪˮ��C�к�����Ԫ�أ������뺬���Ļ����ﷴӦ������CΪ���DΪ���嵥�ʣ�BΪˮ�����Ƴ�DΪ������
�ʴ�Ϊ��
��H2��Fe2O3����2H2O
| ||
�����������ѶȲ������˷�ֹ����ķ��������ָʾ����Ӧ���Լ����ʵ��ƶϣ��������������ѧ��֪ʶ���з����������������������ڻ����Կ����⣮

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ


 �����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�
�����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�