��Ŀ����
ʵ����������һ��ͭ�Ͻ���Ʒ����֪����ƷΪͭп�Ͻ�ͭ���Ͻ�ͭ���Ͻ��ͭ���Ͻ��е�һ�֣�Ϊȷ����Ϊ���ֺϽ��е���һ�֣�ʵ��С���ͬѧ�ǽ��������е�ʵ�飬��ش��������⣺��1��ʵ��������������ͼ��ʾ��ǩ��Ũ���ᣬҪ����20%��ϡ����350�ˣ����Ũ����������______mL��
| ���� �����500mL ��ѧʽ��H2SO4 �ܶȣ�1.4g/cm3 ����������50% |
�ٴ˺Ͻ�Ϊ______�Ͻ�
��д��������Ӧ�Ļ�ѧ����ʽ��______
�۸�����֪����д�������������������x���ı���ʽ��______
������Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������Ϊ______��
��3������ҵ���ú�Cu2S 70%�Ļ�ͭ��lOOt��ͭ��������ұ��������ͭ�ĺϽ𣬣��������ƹ����л�ͭ�����ʧ��Ϊ10%������������ͭ�Ͻ������Ϊ______��
���𰸡���������1�����ݼ�ˮϡ��ǰ�������������䣬��ϡ�ͺ�ϡ���������ҪŨ����������
��2���ٸ������⣬��������������������㷴Ӧ�����������н�����������������ȣ����ÿ��ܽ����Ļ��ϼ������ԭ����������ȷ���Ͻ����ɣ�
�ڸ��ݺϽ�����ȷ������Ӧ�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ��
�۸��ݻ�ѧ����ʽ���������ε�������������������ϵ���г��������������ı���ʽ��
�ܸ��������غ㶨�ɣ�����������Һ��������Ȼ��������ˮ��������Һ������������
��3����������ͭ���������Ͻ���ͭ����������������ɵúϽ��������
����⣺��1�������Ũ��������ΪV
350g×20%=V×1.4g/cm3×50%
V=100mL
��2������Һ���ɺ�õ����崿����32.2g�к������������=98g×20%×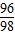 ×100%=19.2g
×100%=19.2g
�������������н���Ԫ�����������������=��32.2g-19.2g����19.2g=65��96���˱�����������п��п��������������ȣ���ˣ����жϺϽ�Ϊͭп�Ͻ�
��ͭп�Ͻ����ϡ�����У�п�����ᷴӦ����ѧ����ʽΪZn+H2SO4�TZnSO4+H2����
����������п32.2gʱ������������x
Zn+H2SO4�TZnSO4+H2��
161 2
32.2g x
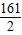 =
=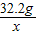
�ܽ����������������=0.4g���μӷ�Ӧп������=32.2g-19.2g=13g
��Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������=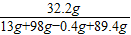 ×100%=16.1%
×100%=16.1%
��4����Cu2S 70%�Ļ�ͭ��lOOt������ͭ������=100t×��1-10%��×70%×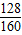 ×100%=50.4t
×100%=50.4t
ͭп�Ͻ���ͭ����������=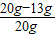 ×100%=35%
×100%=35%
��������ͭ�Ͻ������=50.4t÷35%=144t
�ʴ�Ϊ��
��1��100��
��2����ͭп����Zn+H2SO4�TZnSO4+H2������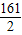 =
=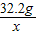 ����16.1%��
����16.1%��
��3��144t��
����������Ϊ���л�ѧ����Ļ㼯��ּ�ڿ���ѧ��������������������������������ʱ����˼·����˳��������յļ��㣮
��2���ٸ������⣬��������������������㷴Ӧ�����������н�����������������ȣ����ÿ��ܽ����Ļ��ϼ������ԭ����������ȷ���Ͻ����ɣ�
�ڸ��ݺϽ�����ȷ������Ӧ�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ��
�۸��ݻ�ѧ����ʽ���������ε�������������������ϵ���г��������������ı���ʽ��
�ܸ��������غ㶨�ɣ�����������Һ��������Ȼ��������ˮ��������Һ������������
��3����������ͭ���������Ͻ���ͭ����������������ɵúϽ��������
����⣺��1�������Ũ��������ΪV
350g×20%=V×1.4g/cm3×50%
V=100mL
��2������Һ���ɺ�õ����崿����32.2g�к������������=98g×20%×
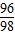 ×100%=19.2g
×100%=19.2g�������������н���Ԫ�����������������=��32.2g-19.2g����19.2g=65��96���˱�����������п��п��������������ȣ���ˣ����жϺϽ�Ϊͭп�Ͻ�
��ͭп�Ͻ����ϡ�����У�п�����ᷴӦ����ѧ����ʽΪZn+H2SO4�TZnSO4+H2����
����������п32.2gʱ������������x
Zn+H2SO4�TZnSO4+H2��
161 2
32.2g x
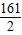 =
=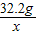
�ܽ����������������=0.4g���μӷ�Ӧп������=32.2g-19.2g=13g
��Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������=
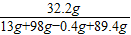 ×100%=16.1%
×100%=16.1%��4����Cu2S 70%�Ļ�ͭ��lOOt������ͭ������=100t×��1-10%��×70%×
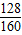 ×100%=50.4t
×100%=50.4tͭп�Ͻ���ͭ����������=
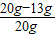 ×100%=35%
×100%=35%��������ͭ�Ͻ������=50.4t÷35%=144t
�ʴ�Ϊ��
��1��100��
��2����ͭп����Zn+H2SO4�TZnSO4+H2������
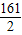 =
=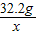 ����16.1%��
����16.1%����3��144t��
����������Ϊ���л�ѧ����Ļ㼯��ּ�ڿ���ѧ��������������������������������ʱ����˼·����˳��������յļ��㣮

��ϰ��ϵ�д�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
�����Ŀ
ʵ����������һ��ͭ�Ͻ���Ʒ����֪����ƷΪͭп�Ͻ�ͭ���Ͻ�ͭ���Ͻ��ͭ���Ͻ��е�һ�֣�Ϊȷ����Ϊ���ֺϽ��е���һ�֣�ʵ��С���ͬѧ�ǽ��������е�ʵ�飬��ش��������⣺
��1��ʵ��������������ͼ��ʾ��ǩ��Ũ���ᣬҪ����20%��ϡ����350�ˣ����Ũ���������� mL��
��1��ʵ��������������ͼ��ʾ��ǩ��Ũ���ᣬҪ����20%��ϡ����350�ˣ����Ũ����������
| ���� �����500mL ��ѧʽ��H2SO4 �ܶȣ�1.4g/cm3 ����������50%��2����20g�Ͻ��ĩ���뵽98g����õ�ϡ�����У�ǡ����ȫ��Ӧ�����ˣ�����Һ���ɺ�õ����崿����32.2g��ͨ������ش� �ٴ˺Ͻ�Ϊ ��д��������Ӧ�Ļ�ѧ����ʽ�� �۸�����֪����д�������������������x���ı���ʽ�� ������Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������Ϊ ��3������ҵ���ú�Cu2S 70%�Ļ�ͭ��lOOt��ͭ��������ұ��������ͭ�ĺϽ𣬣��������ƹ����л�ͭ�����ʧ��Ϊ10%������������ͭ�Ͻ������Ϊ ʵ����������һ��ͭ�Ͻ���Ʒ����֪����ƷΪͭп�Ͻ�ͭ���Ͻ�ͭ���Ͻ��ͭ���Ͻ��е�һ�֣�Ϊȷ����Ϊ���ֺϽ��е���һ�֣�ʵ��С���ͬѧ�ǽ��������е�ʵ�飬��ش��������⣺ |
| ���� �����500mL ��ѧʽ��H2SO4 �ܶȣ�1.4g/cm3 ����������50% |
�ٴ˺Ͻ�Ϊ______�Ͻ�
��д��������Ӧ�Ļ�ѧ����ʽ��______
�۸�����֪����д�������������������x���ı���ʽ��______
������Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������Ϊ______��
��3������ҵ���ú�Cu2S 70%�Ļ�ͭ��lOOt��ͭ��������ұ��������ͭ�ĺϽ𣬣��������ƹ����л�ͭ�����ʧ��Ϊ10%������������ͭ�Ͻ������Ϊ______��
��2011?ƽ������ģ��ʵ����������һ��ͭ�Ͻ���Ʒ����֪����ƷΪͭп�Ͻ�ͭ���Ͻ�ͭ���Ͻ��ͭ���Ͻ��е�һ�֣�Ϊȷ����Ϊ���ֺϽ��е���һ�֣�ʵ��С���ͬѧ�ǽ��������е�ʵ�飬��ش��������⣺
��1��ʵ��������������ͼ��ʾ��ǩ��Ũ���ᣬҪ����20%��ϡ����350�ˣ����Ũ����������______mL��
��2����20g�Ͻ��ĩ���뵽98g����õ�ϡ�����У�ǡ����ȫ��Ӧ�����ˣ�����Һ���ɺ�õ����崿����32.2g��ͨ������ش�
�ٴ˺Ͻ�Ϊ______�Ͻ�
��д��������Ӧ�Ļ�ѧ����ʽ��______
�۸�����֪����д�������������������x���ı���ʽ��______
������Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������Ϊ______��
��3������ҵ���ú�Cu2S 70%�Ļ�ͭ��lOOt��ͭ��������ұ��������ͭ�ĺϽ𣬣��������ƹ����л�ͭ�����ʧ��Ϊ10%������������ͭ�Ͻ������Ϊ______��
��1��ʵ��������������ͼ��ʾ��ǩ��Ũ���ᣬҪ����20%��ϡ����350�ˣ����Ũ����������______mL��
| ���� �����500mL ��ѧʽ��H2SO4 �ܶȣ�1.4g/cm3 ����������50% |
�ٴ˺Ͻ�Ϊ______�Ͻ�
��д��������Ӧ�Ļ�ѧ����ʽ��______
�۸�����֪����д�������������������x���ı���ʽ��______
������Ӧ��������Һ�м���89.4gˮ��������������Һ�е�������������Ϊ______��
��3������ҵ���ú�Cu2S 70%�Ļ�ͭ��lOOt��ͭ��������ұ��������ͭ�ĺϽ𣬣��������ƹ����л�ͭ�����ʧ��Ϊ10%������������ͭ�Ͻ������Ϊ______��
