��Ŀ����
 ��Ҫ����գ�
��Ҫ����գ���1���û�ѧ������д���к��ߣ�
��þԪ��
Mg
Mg
��������������2Fe3+
2Fe3+
���������������Ҫ������SO2
SO2
���ܱ��������Ļ�ѧ���ʵ���С����O2
O2
����2���û�ѧ֪ʶ�ش����������е����⣺
������Ʒ����ʴ���ܺܺã�ԭ����
4Al+3O2=2Al2O3
4Al+3O2=2Al2O3
�����û�ѧ����ʽ��ʾ�������У�A���ƹϣ�B��������C����ͷ��D�����������и��������ʵ���
BD
BD
��������ţ���3����Ԫ�ض��������������������Ҫ���壮
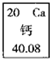
����Ԫ�����ڱ��У���Ԫ�ص���Ϣ��ͼ��ʾ����Ԫ�ص����ԭ������Ϊ
40.08
40.08
���ڸ�Ԫ��Ϊ
����Ԫ��
����Ԫ��
���������Ԫ�ء��ǽ���Ԫ�ء���������������ˮ��Ӧ���˷�Ӧ������
ABC
ABC
��������ĸ��ţ�A������ˮ�� B������ʯ�� C������ʳ�
���������⿼�黯ѧ��������弰��д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӻ��ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ����������ȷ�Ľ�������Ŀ��
����⣺��1���ٸ���Ԫ�ط��ŵ���д������þԪ�ر�ʾΪ��Mg��
�ڸ������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������������ӱ�ʾΪ��2Fe3+��
�������������Ҫ�����Ƕ�����������������+4�۵���Ԫ�غ���-2�۵���Ԫ����ɣ����ݻ��ϼ�ԭ���仯ѧʽ�ɱ�ʾΪ��SO2��
�ܸ��ݱ���������ѧ���ʵ���С�����������ӣ��仯ѧʽΪ��O2��
��2�������ڿ���������������Ӧ������һ�����ܵ���������Ĥ���Ӷ���ֹ���ڲ�������һ����������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2=2Al2O3��
�ڻƹϸ���ά���أ���ͷ�ṩ���࣬�������Ͷ������ṩ�ḻ�ĵ����ʣ���ѡ��BD��
��3���ٸ���Ԫ�����ڱ��е�һ��С�����ṩ����Ϣ��֪����Ԫ�ص����ԭ������Ϊ40.08��
����д�������Ե����ڽ���Ԫ�أ����������⣩���ʸ����ڽ���Ԫ�أ�
����������ˮ��Ӧ������ʯ�ң��ʿ�����������������ջ����е�ˮ�֣���������ȡ��ʯ�ң��÷�Ӧ��ų��������ʿ����ڼ���ʳ�����ABC��
�ʴ�Ϊ����1����Mg����2Fe3+����SO2����O2����2����4Al+3O2=2Al2O3����BD����3����40.08���ڽ���Ԫ�أ���ABC
�ڸ������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������������ӱ�ʾΪ��2Fe3+��
�������������Ҫ�����Ƕ�����������������+4�۵���Ԫ�غ���-2�۵���Ԫ����ɣ����ݻ��ϼ�ԭ���仯ѧʽ�ɱ�ʾΪ��SO2��
�ܸ��ݱ���������ѧ���ʵ���С�����������ӣ��仯ѧʽΪ��O2��
��2�������ڿ���������������Ӧ������һ�����ܵ���������Ĥ���Ӷ���ֹ���ڲ�������һ����������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2=2Al2O3��
�ڻƹϸ���ά���أ���ͷ�ṩ���࣬�������Ͷ������ṩ�ḻ�ĵ����ʣ���ѡ��BD��
��3���ٸ���Ԫ�����ڱ��е�һ��С�����ṩ����Ϣ��֪����Ԫ�ص����ԭ������Ϊ40.08��
����д�������Ե����ڽ���Ԫ�أ����������⣩���ʸ����ڽ���Ԫ�أ�
����������ˮ��Ӧ������ʯ�ң��ʿ�����������������ջ����е�ˮ�֣���������ȡ��ʯ�ң��÷�Ӧ��ų��������ʿ����ڼ���ʳ�����ABC��
�ʴ�Ϊ����1����Mg����2Fe3+����SO2����O2����2����4Al+3O2=2Al2O3����BD����3����40.08���ڽ���Ԫ�أ���ABC
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��Mgԭ���ڻ�ѧ��Ӧ������
��Mgԭ���ڻ�ѧ��Ӧ������