��Ŀ����
11��ȥ�궬�������괺�죬��ʡ������Χ�����ɺ���
��1����Щ��ׯ���ȡ�õ���ˮ���������ˮ��Ӳˮ������ˮ�����õ�������
��2����Щ����ȡ���ǵĿ�ˮ��������ˮ����ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ��ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ��������
��3���������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ
��4������ط���Һ����ɱ��������ʵʩ������˹����꣮Һ����ɱ������ԭ����
��5������ѧ������Щʲô��
��1����Щ��ׯ���ȡ�õ���ˮ���������ˮ��Ӳˮ������ˮ�����õ�������
����ˮ
���ⶨ����ˮ�����ȿ���pH��ֽ����pH�ƣ�
����2����Щ����ȡ���ǵĿ�ˮ��������ˮ����ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ��ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ��������
����
�������ʯӢɳ��С��ʯ��λ�õߵ��Ƿ���ԣ�������
��Ϊʲô�����;�ˮЧ�ʣ�������ٶȼ����ȣ�
����3���������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ
�������
������������Ӳ�Ⱥ�ɱ��ԭ�����4������ط���Һ����ɱ��������ʵʩ������˹����꣮Һ����ɱ������ԭ����
�������Ʋ��б����̬ʱ����Һ���������ɱ�����ʱ�����մ������ȣ�ʹ�Ʋ���ˮ���������С���
����5������ѧ������Щʲô��
��Լ��ˮ����Ϊ��������ˮ������������Ҳ�ɣ�
������һ��������������1������Ӳˮ����ˮ�����ˮ�����������������Բ�ͬ��ȷ��ʹ�÷���ˮ����Ӳˮ����ˮ�����ݼ�����Һ�������ⶨ��Һ���ȵIJ�ͬ��˵���ⶨ����ˮ�����ȿ��õ����ģ�
��2��������ʾ�ļ���ˮ����С��ʯ��ʯӢɳ�ھ�������������ã��Զ��߷���˳������жϣ��������жϵ�ԭ��
��3�����ݾ���Ҫ��Ӳ�Ⱥ�ɱ��ԭ����ж�����ȡ�������ֶεľ���������
��4���Աȿ���Һ����ɱ���������Ĺ�ͬ�㣬����Һ����ɱ������ԭ����
��5������Լ�����Ϊ���������ѧ����Գ����ɺ������������飮
��2��������ʾ�ļ���ˮ����С��ʯ��ʯӢɳ�ھ�������������ã��Զ��߷���˳������жϣ��������жϵ�ԭ��
��3�����ݾ���Ҫ��Ӳ�Ⱥ�ɱ��ԭ����ж�����ȡ�������ֶεľ���������
��4���Աȿ���Һ����ɱ���������Ĺ�ͬ�㣬����Һ����ɱ������ԭ����
��5������Լ�����Ϊ���������ѧ����Գ����ɺ������������飮
����⣺��1��Ӳˮ�к����϶�ĸơ�þ������Ӱ����������ݣ�����÷���ˮ��������Ӳˮ����ˮ��ʹ��pH��ֽ��pH�ƣ����Բⶨ��Һ�����ȣ���˲ⶨ����ˮ�����ȿ���pH��ֽ����pH�ƣ���
��2������С��ʯ����ڵļ�϶�ɳ�ȥˮ�нϴ�Ĺ������ʣ���ʯӢɳ�ļ�϶��С�ɳ�ȥˮ�н�С�Ĺ������ʣ������ھ�ˮ�����˲�Ĺ������ã���ʹ��ʱ�����߲��ɵߵ�ʹ�ã�����ή�;�ˮЧ�ʻ�����ٶȼ����ȣ�
��3��������мȿ�ʹˮ�иơ�þ�����γɳ���������Ӳ��������ɱ��ԭ��������ã���˽���Ӳ�Ⱥ�ɱ��ԭ����ɲ�ȡ������еķ�����
��4����Һ����ɱ��������ʵʩ������˹����꣬������Һ��������ɾ�����ʱ���մ������ȣ���ʹ�Ʋ���ˮ���������С��Σ����Һ����ɱ������ԭ�����������Ʋ��б����̬ʱ����Һ���������ɱ�����ʱ�����մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��5��Ϊ�Կ������ɺ���ͬѧ�ɲ�ȡ��Լ��ˮ�Ĵ�ʩ����Ϊ��������ˮ�������ܼ��ٴ룻
�ʴ�Ϊ��
��1������ˮ��pH��ֽ����pH�ƣ���
��2�����ˣ������ԣ����;�ˮЧ�ʣ�������ٶȼ����ȣ���
��3���������
��4���������Ʋ��б����̬ʱ����Һ���������ɱ�����ʱ�����մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��5����Լ��ˮ����Ϊ��������ˮ������������Ҳ�ɣ���
��2������С��ʯ����ڵļ�϶�ɳ�ȥˮ�нϴ�Ĺ������ʣ���ʯӢɳ�ļ�϶��С�ɳ�ȥˮ�н�С�Ĺ������ʣ������ھ�ˮ�����˲�Ĺ������ã���ʹ��ʱ�����߲��ɵߵ�ʹ�ã�����ή�;�ˮЧ�ʻ�����ٶȼ����ȣ�
��3��������мȿ�ʹˮ�иơ�þ�����γɳ���������Ӳ��������ɱ��ԭ��������ã���˽���Ӳ�Ⱥ�ɱ��ԭ����ɲ�ȡ������еķ�����
��4����Һ����ɱ��������ʵʩ������˹����꣬������Һ��������ɾ�����ʱ���մ������ȣ���ʹ�Ʋ���ˮ���������С��Σ����Һ����ɱ������ԭ�����������Ʋ��б����̬ʱ����Һ���������ɱ�����ʱ�����մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��5��Ϊ�Կ������ɺ���ͬѧ�ɲ�ȡ��Լ��ˮ�Ĵ�ʩ����Ϊ��������ˮ�������ܼ��ٴ룻
�ʴ�Ϊ��
��1������ˮ��pH��ֽ����pH�ƣ���
��2�����ˣ������ԣ����;�ˮЧ�ʣ�������ٶȼ����ȣ���
��3���������
��4���������Ʋ��б����̬ʱ����Һ���������ɱ�����ʱ�����մ������ȣ�ʹ�Ʋ���ˮ���������С��Σ�
��5����Լ��ˮ����Ϊ��������ˮ������������Ҳ�ɣ���
����������Ϊ����ˮ�й�֪ʶ�Ķ�Ƕȿ��飬��Ŀ�ѵ㲻���漰��Ϲ㣬�������ڹ��ɡ��Աȵ�ϰ�߶��ڽ������������нϴ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������ ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������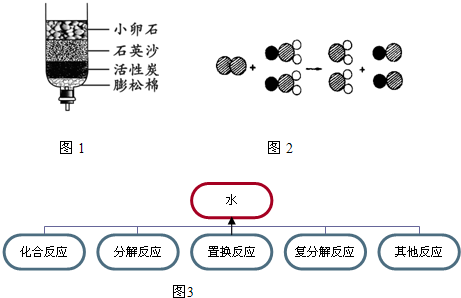
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�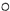 ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ