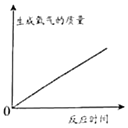
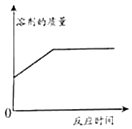
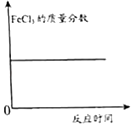
��Ŀ����
����Ŀ����ѧ�����ǵ�����ϢϢ��أ�
��1�������ӵ�ʳ������ۡ��²ˡ����⡢ֲ���͡�ʳ�εȣ����и���������� �� �������л������ ��
��2��������ȱ�ᷢ���������ɣ������ۣ�
��3�������衢�������մɲ豭�����������Ʒ�����������л��ϳɲ��ϵ��� ��
��4������̿�ܳ�ȥ��ͥװ���в������к����壬���������˻���̿���ԣ�
��5������ϴ�Ӽ���ˮ�ܳ�ȥ�·��ϵ����ۣ���������ϴ�Ӽ������ã�
��6�����й���ˮ��˵���������
A.��п��Խ�Ӳˮת��Ϊ��ˮ
B.��ҵ����ֱ���ŷŻ����ˮ��Ⱦ
C.Ѱ�Һ��ʵĴ��������ù���ʹˮ�ֽ�����������ⷽ��
D.����ʹ��ũҩ�����������ũ����������������ˮ��Ⱦ
��7���������ƣ� CaO2�� ��������������ʱ������������ˮ��Ӧ���ɼ��һ�����壬д����Ӧ�Ļ�ѧ����ʽ ��
��8��ijע����ҩҺ�����Ʒ������£� �ٰ� 1.0g ҩƷ����ˮ���Ƴ� 4.0mL ��Һ a��
��ȡ 0.1mL ��Һ a����ˮϡ���� 1.0mL������Һ b��
��ȡ 0.1mL ��Һ b����ˮϡ���� 1.0mL������Һ c��
��ȡ 0.4mL ��Һ c����ˮϡ���� 1.0mL������Һ d��
�������������ƹ�����ҩҺ��ϡ�����ܶȶ��ɽ��ƿ���1g/cm3 �� 1g ��ҩƷ��������Һd �������mL��
���𰸡�
��1����ۣ�ʳ��
��2����/Ca
��3��������
��4������
��5���黯
��6��D
��7��2CaO2+2H2O=2Ca��OH��2+O2��
��8��1000
���������⣺��1������и������ۣ������������ࣻ�����и��������ʣ�ֲ�����и�����֬��ʳ���������Σ���2��������ȱ�ƣ����������������Ͳ��������˻Ỽ��������֢����3�����������л��ϳɲ��ϣ���4������̿�����������ã��ܹ�����ɫ�غ���ζ����5��ʹ��ϴ�Ӽ����ɳ�ȥ���·��ϵ����ۣ�����������ϴ�Ӽ����黯���ܣ���6��A�������еij��ü�����еķ�����Ӳˮת��Ϊ��ˮ����˵����ȷ�� B����ҵ�����к����ж��ɷ֣�ֱ���ŷŻ����ˮ��Ⱦ�Ϳ�����Ⱦ����˵����ȷ��
C������Դ��Ϊ���������е���Դ������ʹ�û��������ʵĹ��������·ֽ�ˮ�Ĵ���������ʹ������⣬���������Ļ������Դ��ѭ����ϵ������Ҫ���ǹ�ֽ��������˵����ȷ��
D������ʹ��ũҩ�����ʻ����ˮ��Ⱦ����˵������7���������ƺ�ˮ��Ӧ�����������ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ��2CaO2+2H2O�T2Ca��OH��2+O2������8����Ϊ��Һ���ܶ���1g/cm3�����Կ�ֱ�ӿ��ɺ������������ʵĿ�����Ȼ���������ʵ���������= ![]() ��100%���㼴�ɣ�
��100%���㼴�ɣ�
���ʵ�����=1.0g��
��Һ������=4.0mL��1g/cm3=4.0cm3��1g/cm3=4.0g��
���ԣ���Һa�����ʵ���������= ![]() ��100%=25%��
��100%=25%��
��Һb����������= ![]() ��100%=2.5%��
��100%=2.5%��
��Һc����������= ![]() ��100%=0.25%��
��100%=0.25%��
��Һd����������= ![]() ��100%=0.1%��
��100%=0.1%��
��1.0gҩƷ����������Ϊx��������ҩҺ����![]() ��100%=0.1%��
��100%=0.1%��
���x=1000g��
�����ܶȿ���1g/mL������Ҳ����1000mL��
���Դ��ǣ���1����ۣ�ʳ�Σ���2����/Ca����3�������裻��4����������5���黯����6��D����7��2CaO2+2H2O=2Ca��OH��2+O2������8��1000��
�����㾫����������Ҫ������Ӳˮ����ˮ��ˮ��Դ����Ⱦ����ε����֪ʶ�㣬��Ҫ����Ӳˮ����ˮ�ļ������÷���ˮ���и�����������ĭ���ٵ���Ӳˮ����ĭ�϶������ˮ��ˮ��Ⱦ:A��ˮ��Ⱦ���ҵ�����ϡ�����������Һ����������ũҩ�����ʵIJ�����ʩ��������ˮ�������ŷ�B����ֹˮ��Ⱦ����ҵ����Ҫ����������ŷš��ᳫ���ŷţ�������ˮҪ���д�������ŷš��ᳫ���ŷţ�����ʩ��ũҩ�����ʣ��ᳫʹ��ũ�ҷʣ���ǿˮ�ʼ�⣮����ˮ��Դ����Լ��ˮ����ֹˮ����Ⱦ������ȷ�����⣮

����Ŀ����ͷ������ȷ���ʳƷ��һ���ص���������������С�ף�����ʹ����ʳƷ�����ɿڣ�С��ѧϰ��ػ�ѧ֪ʶ������������ͷ�õİ�ɫ��ĩ����ʵ��������ȤС���ͬѧһ�����̽����
��1��������̽����ȡ������ɫ��ĩ���Թ��У�������ˮ�������Һ�� pH��7��˵����Һ���ԣ�
��2����������⡿��ɫ��ĩ�ɷ���ʲô�� ���������ϡ�
��̼���ơ�̼�����ƶ���������������ʳƷ��
��̼�����Ʋ��ȶ��������ֽ����ɶ�����̼��ˮ�ȣ� ̼���ƺ��ȶ���
����������衿
�����̼����
�����̼������
��ʵ��̽����������ȤС����Ʋ�ͬʵ�鷽������̽����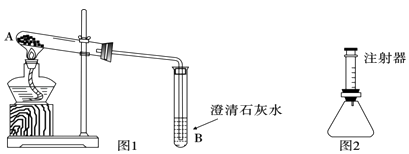
���鷽������ͼ1��ȡ������ɫ��ĩ���Թ��У��������������ʯ��ˮ�����ȣ�����ʯ��ˮ����ǣ��������ȷ��д��װ��B�з�Ӧ�Ļ�ѧ����ʽ ��
���鷽��������ͼ2��ʾװ����Ϊ��Ӧ������ȷ�������������������жϰ�ɫ��ĩ�ijɷ֣�
�ֱ���ʢ��̼���ơ�̼�����ƺͰ�ɫ��ĩ����ƿ�У�ע����������Ũ�ȵ�������ϡ���ᣬ��¼�����
ʵ���� | ��ƿ������[��Դ | ���յõ� CO2 ���/mL | |
���� | ����/g | ||
�� | ̼���� | a | V1 |
�� | ̼������ | a | V2 |
�� | ��ɫ��ĩ | m | V3 |
ʵ��ٵĻ�ѧ��Ӧ����ʽΪ�� ���� m=g�� �����ϱ������ݷ������������ȷ���жϵ������� ��
��3������˼Ӧ�á� �ټ��鷽���У����жϰ�ɫ��ĩ�Ƿ�Ϊ̼���ƺ�̼�����ƵĻ������鷽�������жϣ���������ߵĻ���Ӧ������������� ��
�ڼ���������ͷ�����ʱ������۷��ͣ������л��ᣩ���ټ���������̼�����ƣ�����ʹ��ͷ�����������ԭ���� ��