��Ŀ����
ij��ѧС��ͬѧ���۽ǶȽ�һ�����⸴�ֽⷴӦ��ʵ�ʣ�
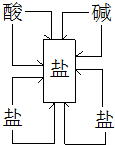
��1�����ֽⷴӦ��ʵ����ijЩ���������ӽ�����ɳ����������______�Ĺ��̣�
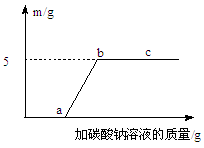
��2�����ݸ��ֽⷴӦ��ʵ�ʣ��γ�һ�����ֽⷴӦ����ͼ����ͼ��ʾ��ͼ��ֱ������������������֮���ܹ�������Ϸ�����Ӧ������ѡ����ʵ�����������ͼ�����ڣ�______��______����ʹ���������ʱ��һ�������������һ���г�����ע������������۵ijɷ���ͬ�����ɣ��������������ɵĻ�ѧ��Ӧ����ʽ������______��

��1�����ֽⷴӦ��ʵ����ijЩ���������ӽ�����ɳ����������______�Ĺ��̣�
��2�����ݸ��ֽⷴӦ��ʵ�ʣ��γ�һ�����ֽⷴӦ����ͼ����ͼ��ʾ��ͼ��ֱ������������������֮���ܹ�������Ϸ�����Ӧ������ѡ����ʵ�����������ͼ�����ڣ�______��______����ʹ���������ʱ��һ�������������һ���г�����ע������������۵ijɷ���ͬ�����ɣ��������������ɵĻ�ѧ��Ӧ����ʽ������______��
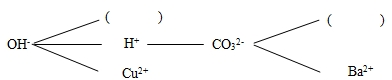
��1�����ݸ��ֽⷴӦ��������������֪�����������ʷ�Ӧʱ������ˮ��������dz������ɣ����ֽⷴӦ�Ͳ��ܷ�����
��2������۵���Ҫ�ɷ�Ϊ̼��ƣ�����Ӧ��ѡ������Ӻ�̼������ӽ�����ɳ��������ܹ����������������������Ϊ笠����ӣ��������ƺ���Σ�������Ȼ�立�Ӧ���ܹ����ɰ�����ˮ���Ȼ��ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��NaOH+NH4Cl�TNaCl+NH3��+H2O��
�ʴ�Ϊ����1��ˮ��
��2��NH4+��Ca2+��NaOH+NH4Cl�TNaCl+NH3��+H2O��
��2������۵���Ҫ�ɷ�Ϊ̼��ƣ�����Ӧ��ѡ������Ӻ�̼������ӽ�����ɳ��������ܹ����������������������Ϊ笠����ӣ��������ƺ���Σ�������Ȼ�立�Ӧ���ܹ����ɰ�����ˮ���Ȼ��ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��NaOH+NH4Cl�TNaCl+NH3��+H2O��
�ʴ�Ϊ����1��ˮ��
��2��NH4+��Ca2+��NaOH+NH4Cl�TNaCl+NH3��+H2O��

��ϰ��ϵ�д�
�����Ŀ