��Ŀ����
��2010?���ޣ���ѧ��ʦл××����ij�о���ѧϰС��һ�����ⶨʵ������һƿ���õ�NaOH�����Ƿ���ʣ���С���ͬѧ����������ʵ��̽������������⡿����I��û���ʣ�ȫ����NaOH��
����II�����ֱ��ʣ��Ⱥ���NaOH���ֺ���Na2CO3��
�������ȫ���ʣ�ȫ����Na2CO3��
���������ϡ�����֪��Ӧ��BaCl2+Na2CO3=2NaCl+BaCO3��
�ڲ�������Һ�ڳ����µ�pH���£�
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС���pH��ֽ�ϣ������ɫ���Աȣ����pH | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
��ʵ����ۡ�ͨ��ʵ�飬˵������������______����ȷ�ģ�
����չ����С��ͬѧΪ�ⶨ����NaOH����ı��ʳ̶ȣ�������������ɫ�������ˡ�ϴ��������Ƶ�������Ϊ2.0g����ԭ�Լ���NaOH����������Ϊ______���ٶ��Լ������������ʣ���
����˼�����õ��������Ʊ��ʵ�ԭ����______���û�ѧ����ʽ��ʾ����
���𰸡��������������������տ����еĶ�����̼���ʣ��ڼ������������Ƿ����ʱ���μ����������ݲ�������Ҫ֤���Dz��ֱ��ʣ����ȼ�����̼���ƣ��ٳ�ȥ̼����֮�������Һ�Լ��ԣ�˵�������������ƣ�
����⣺�������������ֱ��ʣ���Ӧ�����������ƺ�̼�����������ʣ��ڼ�������������ʣ��ʱӦ�ȳ�ȥ̼����֮���ټ�����Һ�Լ��ԣ�
�����������Ƶĺ���ʱ�����˳������ó���������Ȼ����㣮
�ʴ�Ϊ����1��Na2CO3��
��2��NaOH��
��3��������ƽ��
��4����ȥNa2CO3
��5�������
��6������Ʒ�к���̼���Ƶ�����ΪX
BaCl2+Na2CO3=2NaCl+BaCO3��
106 197
X 2.0g
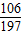 =
=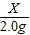
X=1.08g
��ԭ�Լ���NaOH����������Ϊ��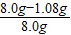 ×100%=86.5%
×100%=86.5%
��7��2NaOH+CO2=Na2CO3+H2O
�������ڼ������������Ƿ��Dz��ֱ���ʱ�������ü�����Һ�Ƿ��Լ�����ȷ������Ϊ̼������ҺҲ�Լ��ԣ�����ֻ���dz�ȥ̼����֮����ȷ����Һ�Լ��ԣ�
����⣺�������������ֱ��ʣ���Ӧ�����������ƺ�̼�����������ʣ��ڼ�������������ʣ��ʱӦ�ȳ�ȥ̼����֮���ټ�����Һ�Լ��ԣ�
�����������Ƶĺ���ʱ�����˳������ó���������Ȼ����㣮
�ʴ�Ϊ����1��Na2CO3��
��2��NaOH��
��3��������ƽ��
��4����ȥNa2CO3
��5�������
��6������Ʒ�к���̼���Ƶ�����ΪX
BaCl2+Na2CO3=2NaCl+BaCO3��
106 197
X 2.0g
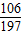 =
=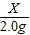
X=1.08g
��ԭ�Լ���NaOH����������Ϊ��
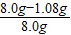 ×100%=86.5%
×100%=86.5%��7��2NaOH+CO2=Na2CO3+H2O
�������ڼ������������Ƿ��Dz��ֱ���ʱ�������ü�����Һ�Ƿ��Լ�����ȷ������Ϊ̼������ҺҲ�Լ��ԣ�����ֻ���dz�ȥ̼����֮����ȷ����Һ�Լ��ԣ�

��ϰ��ϵ�д�
�����Ŀ
