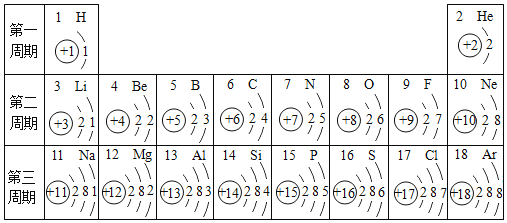
��Ŀ����
���ࡢ��ȡ���������ѧϰ��ѧ�ij��÷�����
��1�����л�ѧ������ʵ�飬Ϊ�˱����о������ǿ��ѻ�ѧʵ�鰴���о�Ŀ�ķ�Ϊ̽�����ʵ����ʡ�̽�����ʵ��Ʊ���̽����Ӧ�����ȡ�
�ݴ�Ӧ������ʵ���е� D ��____________________��Ϊһ�࣬������______________��
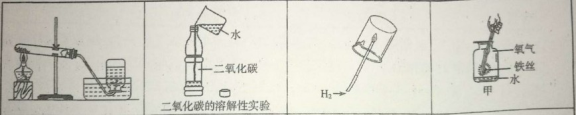
A B C D
��2��C �� CO �dz��л�ѧѧ���ľ��л�ԭ�Ե����ʣ�ͨ��ѧϰ����̼��ԭ������������ͭ�ó��Ľ����ǣ����л�ԭ�Ե����ʲ��뷴Ӧʱ�� ������Ԫ����һ����Ԫ�ػ��ϼ��ڷ�Ӧ��____________������ߡ����ͻ��߲��䡱���������������ۣ����ڷ�Ӧ 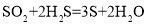 �У� ���л�ԭ�Ե�������___________________________________��
�У� ���л�ԭ�Ե�������___________________________________��
 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д���ȥ���������е����ʣ�������Ϊ���ʣ�����ѡ���Լ���������������ȷ���ǣ�������
ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �������� |
A | Na2SO4��NaOH�� | ϡ���� | �ӹ�����ϡ���ᡢ�����ᾧ |
B | NaC1��Na2CO3�� | CaC12��Һ | ����������CaC12��Һ�����ˡ�����Һ�����ᾧ |
C | KC1��KC1O3�� | MnO2 | ��MnO2�����Ⱥ���ȴ |
D | Fe2��SO4��3��Һ��CuSO4�� | �������� | ���� |
A.A B.B C.C D.D
��ȥ���������е����ʣ���ѡ�õ��Լ���������������ȷ��һ���ǣ� ��
ѡ�� | ���ᴿ������ | ���� | �Լ������� |
A | ����ͭ�� | ľ̿�� | �������������� |
B | ���������� | ����ͭ��ĩ | �ӹ��������� |
C | ������ | ����ʯ | �������� |
D | ˮ | ɫ�� | ����̿ |
A.A B.B C.C D.D
����5���ڱ����ٿ��ˡ�һ��һ·���߷���̳�ᣬ��˿��֮·��һ��ʹ�ҹ����ƾ��Ļ����Ƚ������������磮
��1���Ŵ�˿��֮·�еġ�˿��ָ���Dz�˿����˿�Ļ�ѧ�ɷ��붯��ë�����ƣ����˿���� ������ţ���
A������ | B�������� | C������ | D������ |
��2���ڻ�ҩ���ҹ��Ŵ����Ĵ���֮һ����������ء���ƺ�ľ̿����ɣ��ڻ�ҩ�����ٺ��� ��Ԫ�أ��ڻ�ҩ��ըʱ�����ķ�ӦΪ��S+2KNO3+3C�TX+3CO2��+N2������X�Ļ�ѧʽΪ ��
��3���ҹ��ĸ��������������ȣ������ֹ���������������ڸ����·����û���Ӧʵ���캸�ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��


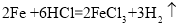
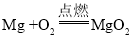
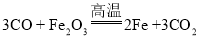
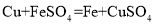
 �Ż��������� B.
�Ż��������� B. ���ʯ���Բò���
���ʯ���Բò��� �û���̿��ˮ D.
�û���̿��ˮ D. �ý��ʯ����ʯ
�ý��ʯ����ʯ