��Ŀ����
��1���û�ѧ������գ�
����ԭ�ӵĽṹʾ��ͼ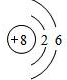
 �� ��3����������
�� ��3����������
�����������������
�� ����
��2��д�����š�2O3�������ֵĺ��壺�١�2����ʾ
��3�����������ߺš��ɴ�����̫�յ��ǡ���������F�����ػ������������з����ķ�ӦΪC2H8N2+2R�T3N2+4H2O+2CO2������R�Ļ�ѧʽ��
��4��������������������ȼ�գ��÷�Ӧ��������������ˮ����������
��5���ڷ�Ӧ2X+Y2=2Z�У���֪X����Է�������Ϊ24��Y2����Է�������Ϊ32����Z����Է�������Ϊ
��6���ڻ�ѧ��ӦA+2B=C�У�1.5gA��������B��ַ�Ӧ����9.5gC����μӷ�Ӧ��B������Ϊ
����ԭ�ӵĽṹʾ��ͼ


3Fe2+
3Fe2+
�� ��������Al2��SO4��3
Al2��SO4��3
�������������������
OH-
OH-
�� ��+2�۵ĸ�Ԫ��| +2 |
| Ca |
| +2 |
| Ca |
He
He
����2��д�����š�2O3�������ֵĺ��壺�١�2����ʾ
2����������
2����������
���ڡ�3����ʾһ�����������к���3����ԭ��
һ�����������к���3����ԭ��
����3�����������ߺš��ɴ�����̫�յ��ǡ���������F�����ػ������������з����ķ�ӦΪC2H8N2+2R�T3N2+4H2O+2CO2������R�Ļ�ѧʽ��
N2O4
N2O4
����4��������������������ȼ�գ��÷�Ӧ��������������ˮ����������
1��8��9
1��8��9
����5���ڷ�Ӧ2X+Y2=2Z�У���֪X����Է�������Ϊ24��Y2����Է�������Ϊ32����Z����Է�������Ϊ
40
40
����6���ڻ�ѧ��ӦA+2B=C�У�1.5gA��������B��ַ�Ӧ����9.5gC����μӷ�Ӧ��B������Ϊ
8
8
g����������1������ԭ�ӵĺ���������Ϊ8��������ԭ�ӽṹʾ��ͼ���ɣ�
�ڸ������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������д��
�۸��ݻ����ﻯѧʽ����д����������д���ɣ�
�����������е������������������ӣ�д�������ӷ��ż��ɣ�
�ݸ���Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�����д��
���ݵ��ʻ�ѧʽ����д����������д���ɣ�
��2�����ڷ��ӷ���ǰ������ֱ����ӵĸ��������ڻ�ѧʽ��Ԫ�����½ǵ����ֱ�ʾһ������������ԭ�ӵ���Ŀ��
��3���������غ㶨�ɣ���Ӧǰ��ԭ�����ࡢ��Ŀ�����䣬�ݴ��ɷ�Ӧ�Ļ�ѧ����ʽ�ƶϷ�Ӧ��R�Ļ�ѧʽ��
��4�����ݻ�ѧ����ʽ�и����ʵ������ȵ�����Է��������͵ıȽ��з������
��5���ڷ�Ӧ2X+Y2=2Z�У���Z����Է�������Ϊx�����������غ㶨�ɽ��з������
��6���������غ㶨�ɽ��з������
�ڸ������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�������д��
�۸��ݻ����ﻯѧʽ����д����������д���ɣ�
�����������е������������������ӣ�д�������ӷ��ż��ɣ�
�ݸ���Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�����д��
���ݵ��ʻ�ѧʽ����д����������д���ɣ�
��2�����ڷ��ӷ���ǰ������ֱ����ӵĸ��������ڻ�ѧʽ��Ԫ�����½ǵ����ֱ�ʾһ������������ԭ�ӵ���Ŀ��
��3���������غ㶨�ɣ���Ӧǰ��ԭ�����ࡢ��Ŀ�����䣬�ݴ��ɷ�Ӧ�Ļ�ѧ����ʽ�ƶϷ�Ӧ��R�Ļ�ѧʽ��
��4�����ݻ�ѧ����ʽ�и����ʵ������ȵ�����Է��������͵ıȽ��з������
��5���ڷ�Ӧ2X+Y2=2Z�У���Z����Է�������Ϊx�����������غ㶨�ɽ��з������
��6���������غ㶨�ɽ��з������
����⣺��1������ԭ�ӵĺ���������Ϊ8��������2�����Ӳ㣬��һ������2�����ӣ��ڶ�������6�����ӣ���ԭ�ӽṹʾ��ͼΪ ��
��
�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�3���������ӿɱ�ʾΪ��3Fe2+��
������������Ԫ����+3�ۣ��������-2�ۣ��仯ѧʽΪAl2��SO4��3��
�����������е������������������ӣ������ӷ���Ϊ��OH-��
����Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�+2�۵ĸ�Ԫ�ؿɱ�ʾΪ��
��
��������ϡ�����嵥�ʣ�ֱ����Ԫ�ط��ű�ʾ�仯ѧʽ���仯ѧʽΪHe��
��2�����ڷ��ӷ���ǰ������ֱ����ӵĸ��������ڻ�ѧʽ��Ԫ�����½ǵ����ֱ�ʾһ������������ԭ�ӵ���Ŀ����2O3�������ֵĺ��壺�١�2����ʾ2���������ӣ��ڡ�3����ʾһ�����������к���3����ԭ�ӣ�
��3�����ݷ�Ӧ�Ļ�ѧ����ʽC2H8N2+2R�T3N2+4H2O+2CO2����Ӧ����̼���⡢��ԭ�Ӹ����ֱ�Ϊ2��8��2����Ӧ�����������̼���⡢������ԭ�Ӹ����ֱ�Ϊ2��8��6��8�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��2R�к���4����ԭ�Ӻ�8����ԭ�ӣ���ÿ��R������2����ԭ�Ӻ�4����ԭ�ӹ��ɣ�������R�Ļ�ѧʽΪN2O4��
��4��������������������ȼ�յĻ�ѧ����ʽΪ��2H2+O2
2H2O���÷�Ӧ��������������ˮ����������4��32��36=1��8��9��
��5���ڷ�Ӧ2X+Y2=2Z�У���֪X����Է�������Ϊ24��Y2����Է�������Ϊ32����Z����Է�������Ϊx����24��2+32=2x��x=40��
��6���ڻ�ѧ��ӦA+2B=C�У�1.5gA��������B��ַ�Ӧ����9.5gC����μӷ�Ӧ��B������Ϊ9.5g-1.5g=8g��
�ʴ�Ϊ����1���� ����3Fe2+����Al2��SO4��3����OH-����
����3Fe2+����Al2��SO4��3����OH-����
����He��
��2��2���������ӣ�һ�����������к���3����ԭ��
��3��N2O4����4��1��8��9����5��40����6��8��
 ��
���������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�3���������ӿɱ�ʾΪ��3Fe2+��
������������Ԫ����+3�ۣ��������-2�ۣ��仯ѧʽΪAl2��SO4��3��
�����������е������������������ӣ������ӷ���Ϊ��OH-��
����Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�+2�۵ĸ�Ԫ�ؿɱ�ʾΪ��
| +2 |
| Ca |
��������ϡ�����嵥�ʣ�ֱ����Ԫ�ط��ű�ʾ�仯ѧʽ���仯ѧʽΪHe��
��2�����ڷ��ӷ���ǰ������ֱ����ӵĸ��������ڻ�ѧʽ��Ԫ�����½ǵ����ֱ�ʾһ������������ԭ�ӵ���Ŀ����2O3�������ֵĺ��壺�١�2����ʾ2���������ӣ��ڡ�3����ʾһ�����������к���3����ԭ�ӣ�
��3�����ݷ�Ӧ�Ļ�ѧ����ʽC2H8N2+2R�T3N2+4H2O+2CO2����Ӧ����̼���⡢��ԭ�Ӹ����ֱ�Ϊ2��8��2����Ӧ�����������̼���⡢������ԭ�Ӹ����ֱ�Ϊ2��8��6��8�����ݷ�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬��2R�к���4����ԭ�Ӻ�8����ԭ�ӣ���ÿ��R������2����ԭ�Ӻ�4����ԭ�ӹ��ɣ�������R�Ļ�ѧʽΪN2O4��
��4��������������������ȼ�յĻ�ѧ����ʽΪ��2H2+O2
| ||
��5���ڷ�Ӧ2X+Y2=2Z�У���֪X����Է�������Ϊ24��Y2����Է�������Ϊ32����Z����Է�������Ϊx����24��2+32=2x��x=40��
��6���ڻ�ѧ��ӦA+2B=C�У�1.5gA��������B��ַ�Ӧ����9.5gC����μӷ�Ӧ��B������Ϊ9.5g-1.5g=8g��
�ʴ�Ϊ����1����
 ����3Fe2+����Al2��SO4��3����OH-����
����3Fe2+����Al2��SO4��3����OH-����| +2 |
| Ca |
��2��2���������ӣ�һ�����������к���3����ԭ��
��3��N2O4����4��1��8��9����5��40����6��8��
�����������ѶȲ������ճ�����ѧ�������д�����������غ㶨�ɲ��������������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ
 ��ѧ�����Ǹ߶�Ũ���ķ�����ϵ����ȷ���ļ�¼�����������ѧ��Ϣ��
��ѧ�����Ǹ߶�Ũ���ķ�����ϵ����ȷ���ļ�¼�����������ѧ��Ϣ�� ����ʾ������������
����ʾ������������