��Ŀ����
�������ճ������еı���Ʒ����ͨ����ĥ���������ݼ���ճ�ϼ�����ɡ�ĥ�������������Ҫ�ɷ֣�Լռ����������һ�����ϡ�Ϊ�˲ⶨһ����̼���Ϊĥ������������̼��Ƶĺ�����ͬѧ�Ǹ���̼��Ƶ�����������������ֲ�ͬ�ķ���(��̼����⣬���������е��������ʲ������ᷴӦ�������壬�Ҿ�����ˮ)��
����һ����һ�������������ˮ�г�ֽ��裬Ȼ�����X��ϴ�ӡ�����Ȳ�������������������������ټ����̼��Ƶ�����������
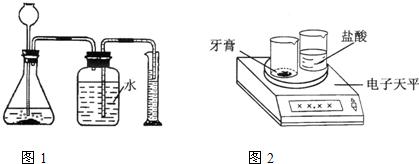
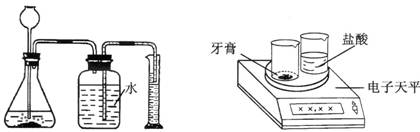
����������һ��������������������ᷴӦ����ͼ1��ʾװ��(װ�õ�����������)������������̼��������(��ƿ��Һ��߶ȱ仯���Բ���)�����ø������¶�����̼���ܶȣ����������̼��������Ȼ����ݻ�ѧ����ʽ���̼��Ƶ��������ټ����̼��Ƶ�����������
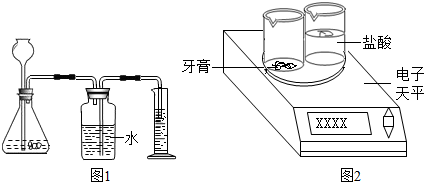
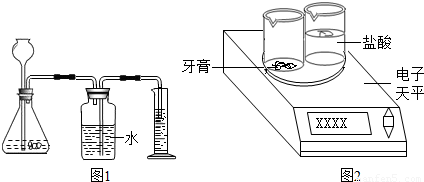
����������ͼ2��ʾװ�ã���һ��������������������ᷴӦ������������ݣ������������̼�����������Ȼ����ݻ�ѧ����ʽ���̼��Ƶ��������ټ����̼��Ƶ�����������
����һ����һ�������������ˮ�г�ֽ��裬Ȼ�����X��ϴ�ӡ�����Ȳ�������������������������ټ����̼��Ƶ�����������
����������һ��������������������ᷴӦ����ͼ1��ʾװ��(װ�õ�����������)������������̼��������(��ƿ��Һ��߶ȱ仯���Բ���)�����ø������¶�����̼���ܶȣ����������̼��������Ȼ����ݻ�ѧ����ʽ���̼��Ƶ��������ټ����̼��Ƶ�����������
����������ͼ2��ʾװ�ã���һ��������������������ᷴӦ������������ݣ������������̼�����������Ȼ����ݻ�ѧ����ʽ���̼��Ƶ��������ټ����̼��Ƶ�����������
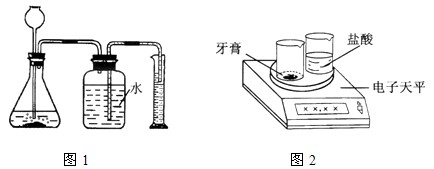
ͼ1ͼ2��ʦ������ַ����ṩ�������������Ʒ��ͬѧ�ǽ��з���ʵ�顣ʵ���У�ͬѧ�Dz����淶������������Ҫ��ش��������⣺
(1)����һ�в���X��___________�����շ���һ����ʵ���ͬѧ�ڹ涨ʱ����û�����ü����ʵ�飬��д�����ܵ�ԭ��_________________��________________����д��������
(2)̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ��___________________����������ʵ����ƫ�ͣ�д��һ�����ܵ�ԭ��_________________����ĸĽ������ǣ�________________��
(3)��������ʵ����ƫ�ߣ�ԭ������ǣ�_______________________��
(1)����һ�в���X��___________�����շ���һ����ʵ���ͬѧ�ڹ涨ʱ����û�����ü����ʵ�飬��д�����ܵ�ԭ��_________________��________________����д��������
(2)̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ��___________________����������ʵ����ƫ�ͣ�д��һ�����ܵ�ԭ��_________________����ĸĽ������ǣ�________________��
(3)��������ʵ����ƫ�ߣ�ԭ������ǣ�_______________________��
(1)���ˣ���ճ�ϼ������ܽ������з��ݼ�����������ĭ���¹���ʱ�䳤
(2)CaCO3+2HCl==CaCl2+H2O+CO2����CO2����ˮ����ƿ�м�һ����
(3)H2O��HCl�Ļӷ�
(2)CaCO3+2HCl==CaCl2+H2O+CO2����CO2����ˮ����ƿ�м�һ����
(3)H2O��HCl�Ļӷ�

��ϰ��ϵ�д�
�����Ŀ