��Ŀ����
������ͼ��ʾʵ��װ�ã���ش���������

��1��ͼ��e��f���������ƣ�e ��f ��
��2��������غͶ������̹�����������ѡ�õķ���װ���� ������ĸA��B��C��D������Ӧ�Ļ�ѧ����ʽ�� ����Ӧǰ�������̵����� �������䡱���С������
��3�� �ÿ�״�����Һ�峣������ȡ����ʱ����Cװ�ã�����������ſ�״���壩����Bװ�õ��ŵ��� ���۲�ͼCװ�ã�������ȡ���� ������С���ֹͣ�����С�
��4��ʵ��������˫��ˮ�Ͷ������̷�Ӧ�������Ļ�ѧ��Ӧ����ʽΪ ����ʵ�� ����ܡ����ܡ���ѡ��Cװ�������Ʒ�Ӧ������ֹͣ������Dװ�����ռ����ɵ�����������Ӧ�� ����g��h��ͨ�롣

��1��ͼ��e��f���������ƣ�e ��f ��
��2��������غͶ������̹�����������ѡ�õķ���װ���� ������ĸA��B��C��D������Ӧ�Ļ�ѧ����ʽ�� ����Ӧǰ�������̵����� �������䡱���С������
��3�� �ÿ�״�����Һ�峣������ȡ����ʱ����Cװ�ã�����������ſ�״���壩����Bװ�õ��ŵ��� ���۲�ͼCװ�ã�������ȡ���� ������С���ֹͣ�����С�
��4��ʵ��������˫��ˮ�Ͷ������̷�Ӧ�������Ļ�ѧ��Ӧ����ʽΪ ����ʵ�� ����ܡ����ܡ���ѡ��Cװ�������Ʒ�Ӧ������ֹͣ������Dװ�����ռ����ɵ�����������Ӧ�� ����g��h��ͨ�롣
(1) �Թ� ����©�� (2) A 2KClO3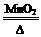 2KCl + 3O2 �� ����
2KCl + 3O2 �� ����
(3) �濪���ã������ͣ����ʱ���Ʒ�Ӧ�ķ�����ֹͣ�� ����
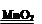 ��4) 2H2O2 2H2O + O2 �� ���� g
��4) 2H2O2 2H2O + O2 �� ���� g
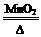 2KCl + 3O2 �� ����
2KCl + 3O2 �� ���� (3) �濪���ã������ͣ����ʱ���Ʒ�Ӧ�ķ�����ֹͣ�� ����
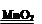 ��4) 2H2O2 2H2O + O2 �� ���� g
��4) 2H2O2 2H2O + O2 �� ���� g�����������1������ʵ���ҳ��������ش�ͼ������e���������Թܣ�����f�������dz���©����
��2��������غͶ������̹�������������������ڶ��������������ͼ��ȵ������£��ֽ������Ȼ��غ��������ʷ�Ӧ�Ļ�ѧ����ʽ��2KClO3
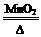 2KCl+3O2����������Ҫ����������͡�������ȡװ�ã���ѡA�����ڶ��������Ǹ÷�Ӧ�Ĵ������ʷ�Ӧǰ�������̵��������䡣
2KCl+3O2����������Ҫ����������͡�������ȡװ�ã���ѡA�����ڶ��������Ǹ÷�Ӧ�Ĵ������ʷ�Ӧǰ�������̵��������䡣��3������Cװ�����и����ĸ��壬��������Һ������ã���ʹ�ø���װ����ȡ�����ͻ���ŵ��ǣ�������ʱ���Ʒ�Ӧ������ֹͣ������ͼCװ���еĹ�Һ������һ�����Է�Ӧ���ڽ��С�
��4�����������ڶ������̵Ĵ������£��ֽ�����ˮ���������ʷ�Ӧ�Ļ�ѧ����ʽ��2H2O2
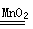 2H2O+O2�������ڶ��������Ƿ�ĩ״�ģ���������������ſ�״���塱������ʵ�ֹ�Һ����룬�ʲ���ѡ��Cװ�������Ʒ�Ӧ������ֹͣ�������������ܶȱȿ��������������ſ������ռ�������װ��D���С��ſ��������ռ�������������Ӧ�ӽϳ��ĵ���g���룬���������Ͻ������ӽ϶̵ĵ����ų���
2H2O+O2�������ڶ��������Ƿ�ĩ״�ģ���������������ſ�״���塱������ʵ�ֹ�Һ����룬�ʲ���ѡ��Cװ�������Ʒ�Ӧ������ֹͣ�������������ܶȱȿ��������������ſ������ռ�������װ��D���С��ſ��������ռ�������������Ӧ�ӽϳ��ĵ���g���룬���������Ͻ������ӽ϶̵ĵ����ų������������������ʵ�����Ʒ���ԭ���Ͳ���ע������ǽ���Ĺؼ������⣬������ص��dz�������ķ���װ�ú��ռ�װ�õ�ѡȡ������Ҫѧ����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����װ�ã�������ȡ������ܽ��Ժ��ܶȵĴ�Сѡ���ռ�����ķ�����

��ϰ��ϵ�д�
�����Ŀ









