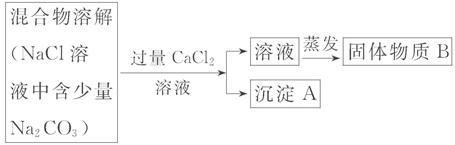
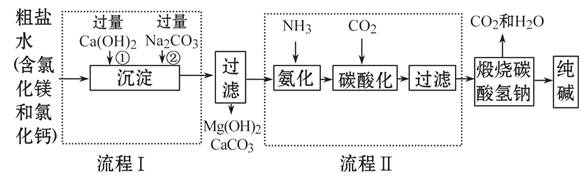
��Ŀ����
��1����ѧ������������أ�����Ϊ�˸�С������Ӫ�����ƶ��������ʾ��ʳ�ף�
a��������Ҫ���е�Ӫ������ ������ࡱ����֬������Ϊ��ʹӪ�����⣬�㽨��Ӧ�����ӵ�ʳ���� ����߲ˡ����ȳ�����
b��С�������ڳ����õ���������Ʒ�У���Ҫ�������ںϳɲ��ϵ��� ������ţ�
������ ����Χȹ ���������ף�
c����ij��У�С��ͬѧ��С�ı�����ҧ�ˣ����϶�Һ�����ԣ�������������������ͿĨ���Լ���ʹ����ǣ�����ĸ��
A��ʳ�� B������Һ C����ˮ
��2��С���ڲ�������ʱ��������һ�λ���������������ʹ�Ϻ�ɫKMnO4��Һ�����ɫ��Һ����Ӧ����ʽΪ��5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2 ��
��
�ٷ���ʽ�����һ�����ʵĻ�ѧʽӡˢ�������С�������������˽��������һ�ֳ������ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ�� �����������MnԪ�ػ��ϼ�Ϊ ��5SO2�С�5���ĺ����� ��
����Ϣ��ʾ�����������K+��SO42-���ɣ��������Һ����ɫ������Һ����ɫͨ�����ɹ����������Ӿ����ģ��������ϼ���Ϣ��ʾ��KMnO4��Һ���ֵ��Ϻ�ɫ�������� �������ӷ��ţ����ӱ��ֳ����ģ�
��3���ֽ�H2SO4��Ba(OH)2��Na2CO3��������ͬʱ���뵽������ˮ�У���ַ�Ӧ����ˣ��ð�ɫ����B����ɫ��ҺA�����ⶨ��ҺpH��7��
��Ϣ�������ᱵ������ˮҲ�������� ��̼�ᱵ������ˮ������������

�ݴ��жϣ���ҺA��һ������������ ��ɫ����B���� ����������������
| ��ʳ | �� |
| ��ʳ | �����⡢�Ǵ��㡢�Ź��� |
| ���� | �Ӹ�ţ�� |
a��������Ҫ���е�Ӫ������ ������ࡱ����֬������Ϊ��ʹӪ�����⣬�㽨��Ӧ�����ӵ�ʳ���� ����߲ˡ����ȳ�����
b��С�������ڳ����õ���������Ʒ�У���Ҫ�������ںϳɲ��ϵ��� ������ţ�
������ ����Χȹ ���������ף�
c����ij��У�С��ͬѧ��С�ı�����ҧ�ˣ����϶�Һ�����ԣ�������������������ͿĨ���Լ���ʹ����ǣ�����ĸ��
A��ʳ�� B������Һ C����ˮ
��2��С���ڲ�������ʱ��������һ�λ���������������ʹ�Ϻ�ɫKMnO4��Һ�����ɫ��Һ����Ӧ����ʽΪ��5SO2+2KMnO4+2H2O�TK2SO4+2MnSO4+2
 ��
���ٷ���ʽ�����һ�����ʵĻ�ѧʽӡˢ�������С�������������˽��������һ�ֳ������ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ�� �����������MnԪ�ػ��ϼ�Ϊ ��5SO2�С�5���ĺ����� ��
����Ϣ��ʾ�����������K+��SO42-���ɣ��������Һ����ɫ������Һ����ɫͨ�����ɹ����������Ӿ����ģ��������ϼ���Ϣ��ʾ��KMnO4��Һ���ֵ��Ϻ�ɫ�������� �������ӷ��ţ����ӱ��ֳ����ģ�
��3���ֽ�H2SO4��Ba(OH)2��Na2CO3��������ͬʱ���뵽������ˮ�У���ַ�Ӧ����ˣ��ð�ɫ����B����ɫ��ҺA�����ⶨ��ҺpH��7��
��Ϣ�������ᱵ������ˮҲ�������� ��̼�ᱵ������ˮ������������

�ݴ��жϣ���ҺA��һ������������ ��ɫ����B���� ����������������
��1��a�ǣ��߲ˣ�b��;c��B;��2����H2SO4;+7;5������������ӣ���MO4+;��3��H+��Na+��SO42-��H2O;�����
�����������1��a��������Ҫ���е�Ӫ�������࣬Ϊ��ʹӪ�����⣬Ӧ�����ӵ�ʳ�����߲ˣ��Բ���ά���أ�b���ڳ����õ�����Ʒ�У���Ҫ�������ںϳɲ��ϵ���������c����ij��У�С��ͬѧ��С�ı�����ҧ�ˣ����϶�Һ�����ԣ�����������ʳ��ͿĨ���Լ���ʹ��ģ���2���ٷ���ʽ�����һ�����ʵĻ�ѧʽӡˢ�������С�������������˽��������һ�ֳ������ᣬ���������غ㶨���Ʋ��仯ѧʽH2SO4; �����������MnԪ�ػ��ϼ�Ϊ+7�ۣ�5SO2�С�5���ĺ�����5������������ӣ��������е�������֪KMnO4��Һ���ֵ��Ϻ�ɫ��������MO4+���ӱ��ֳ�����;��3���������е��жϣ���ҺAΪ�����ƺ�����Ļ��Һ������Һ��һ�����������У�)H+��Na+��SO42-��H2O; ��ɫ����B��̼�ᱵ�����ڴ�����

��ϰ��ϵ�д�
 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ
 CO2
CO2 2H2����O2��
2H2����O2�� 2Fe��3CO2
2Fe��3CO2