��Ŀ����
����Ŀ��������ͼŨ������̱꣬�ش��������⣺
��1��ij�о�С���ͬѧ���ø���������100g10%��ϡ���ᣬ������� g������һλС����������ʱӦѡ�� ���10mL����50mL������Ͳ��ȡ��
��2��С��ͬѧ��������ϡ����������ˮ������Ҫ�ɷ�ΪCaCO3��Mg��OH��2����CaCO3������������ȡ10.0gˮ������������100g10%��ϡ�����У���ø�ʱ�����������������������ʾ��ͨ��ʵ�飬�����ˮ����CaCO3������������

���𰸡���1��27.0��50mL����2��75%
����������1������ϡ��ʱ���ʵ�������������������������������ѡ��������Ͳ��
��2���������е�֪ʶ���з�����̼����������ᷴӦ���ɶ�����̼����������þ�����ᷴӦ���ɵ�ʱ�Ȼ�þ��ˮ�����������壬���ݱ����ṩ�����ݼ������̼��Ƶ��������Ӷ����̼��Ƶĺ�����
�⣺��1������Ũ��Һ������Ũ��Һ����������=ϡ��Һ������ϡ��Һ������������
������������=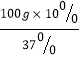 =27.0g�����Ϊ27.0g��1.18g/cm3=23cm3=23mL��ѡ��50mL����Ͳ��
=27.0g�����Ϊ27.0g��1.18g/cm3=23cm3=23mL��ѡ��50mL����Ͳ��
��2��̼����������ᷴӦ���ɶ�����̼����������þ�����ᷴӦ�����ɶ�����̼���������������ٸı䣬˵��̼����Ѿ���Ӧ��ȫ������4���Ӻ�Ӧ����ȫ��
������3.3g������̼��Ҫ̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 3.3g![]()
x=7.5g
��ˮ����CaCO3����������Ϊ��![]() ��100%=75%
��100%=75%
�𣺣�1��27.0��50mL��
��2���𣺸�ˮ����CaCO3����������Ϊ75%��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�